We can now alter the genomes of invasive species to slow their advance. Should we?
By Alison Hawkes
(Illustration: Jason Holley)
Every year, as summer turns to fall, the mouse population on the South Farallon Islands explodes to plague-like densities, numbering 490 mice per acre, among the highest found on any island in the world. The scientists who live and work there describe the assault of the invasive house mouse as a kind of purgatory in the otherwise stunning, windswept smattering of rocky islets and sea stacks 30 miles outside the Golden Gate.
“At night they would be everywhere,” says Peter Pyle, a wildlife biologist who spent more than 20 fall seasons living at the research station on Southeast Farallon Island. “I had them crawling on top of me at night and in my hair. I tried to mouse-proof the house but we’d catch 50 mice in the night.”
Besides making scientific research on the Farallones a harrowing experience, the common house mouse, Mus musculus, has substantially disrupted the island ecosystem — spreading the seeds of invasive plants, eating the endemic Farallon camel cricket as well as a species of daisy called maritime goldfields that provides critical nesting material for birds, and indirectly causing the demise of the island’s breeding population of ashy storm- petrels, a California bird of special concern.
It’s a familiar story on islands all over the world where rodents — prolific feeders and breeders — are a leading cause of extinctions. Massive efforts have been undertaken to kill invasive rodents and usually involve broadcasting rodenticide; other options, like trapping mice or releasing biological controls in the form of snakes or cats, have been ineffective.
“At night they would be everywhere. I had them crawling on top of me at night and in my hair. I tried to mouse-proof the house but we’d catch 50 mice in the night.”
In 2011, the United States Fish and Wildlife Service initiated a plan to exterminate the Farallones mice. The proposed plan, should it go forward, includes deploying helicopters to spray a rodenticide-laced bait throughout the steep, rugged terrain — on the nine problematic southern islands comprising 121 acres — to reach all the mouse burrows. For the treatment to succeed every mouse must be exterminated; a single pregnant female could lead to the quick re-generation of the population. The agency addressed the risks — non-target species might eat the bait, mouse predators might be poisoned, toxins might drift into the marine environment, helicopters could disturb birds — and came up with a plan to mitigate them. Although scientists have been largely supportive of the plan, a vocal segment of the public has come out against using poison on wilderness lands and causing the mice to suffer. The project now sits in limbo because of lapses in federal funding; in the meantime the mice keep eating and procreating.
But what if there was another way to do this dirty work?
At North Carolina State University, neurobiologist John Godwin was scrolling through his news feed and came across an item about the Farallones mouse project as the rodenticide plan was first announced. Godwin works with Mus musculus as part of his research into animal behavior, and he’s long been interested in the genetic divergence among mice that have stowed away to islands. It struck him there might be a genetic solution to the Farallones mouse problem, or, if not there, on some other island suffering its own version of mouse Armageddon. “I thought, ‘This is the kind of place you would probably want to go — a remote island surrounded by white sharks with very strictly controlled access,’” says Godwin, a former field ecologist.
At NC State, Godwin had colleagues working at the cutting edge of genomics. Entomologist Fred Gould has been bioengineering a mosquito whose modified genes prevent it from transmitting malaria and dengue fever. And David Threadgill, a geneticist now at Texas A&M, studies cancer using house mice as models.
Fortuitously, Mus musculus is not only one of the most widely distributed mammals on Earth but a longtime staple of laboratory research, and, in 2002, it had become the second mammal, after humans, to have its genome decoded. As a result, scientists know a lot about what makes a house mouse tick, genetically and behaviorally. Godwin and his colleagues began discussing a novel research question: Could they genetically engineer a house mouse that would breed itself out of existence if set loose on an island?
The first step was to contact USFWS’s refuge manager for the Farallones. But in a conference call it became apparent that the agency wanted a faster and more straightforward method for solving the islands’ mouse problem, according to Threadgill. Still, the conference call introduced the NC State team to Karl Campbell, the program director for Island Conservation, a Santa Cruz-based non-profit that specializes in ridding islands of invasive species. IC was involved in the Farallones mouse project in its early stages.
Campbell perked up at the prospect of developing a bioengineered mouse for island eradication work; he had already been thinking along those lines.“I knew it was potentially possible, but I didn’t have any contacts in the field,” Campbell recalls. “I was all over it pretty quickly.”

(Illustration: Donna Almendrala)
Scientists have long dreamed of controlling species by tinkering with their genes, but it’s only in recent years that advances in molecular biology have supplied them with a surefire method. Arguably the biggest development has been the discovery of a technique for editing genes at precise locations called CRISPR-Cas9, pioneered in 2012 by University of California-Berkeley molecular biochemist Jennifer Doudna.
Besides raising hopes of curing cancer and congenital diseases and the specter of “designer babies,” CRISPR-Cas9 has scientists talking about everything from ending world hunger with drought-resistant crops to putting a stop to malaria by creating disease-proof mosquitoes, or even mitigating climate change with biofuel-producing yeast.
Whether or not the technology delivers in all these cases, CRISPR-Cas9 is a major scientific breakthrough that provides a cheap and easy means to alter almost any gene in any sexually reproducing species — which is both exciting and scary, considering that the ethics of deploying it have not been ironed out.
The field of conservation has been similarly intrigued by the possibilities. Imagine re-designing an endangered species to give it a better chance of survival. Or how about bringing back an extinct species like the woolly mammoth, or some approximation of it, by inserting genes recovered from frozen specimens into an elephant genome? Or take an invasive species like the house mouse on the Farallones — what if it could be humanely exterminated by engineering a male that can’t produce female offspring?
These aren’t theoretical scenarios anymore. In places around the world, scientists are pursuing such projects, though they are in the early stages. Along with creating enough safeguards so that the risks associated with unleashing a bioengineered species into the wild don’t surpass the benefits, scientists need to gain society’s approval, and that of the regulators as well. After all, genetically modified organisms already evoke the public’s ire when it comes to food crops and pests.
Ryan Phelan and her husband Stewart Brand, the co-founders of Revive & Restore, a project of the San Francisco-based Long Now Foundation, have been trying to catalyze the “genetic rescue” of species as an outgrowth of their work to bring back the woolly mammoth, passenger pigeon, and other extinct species. “It’s gathering momentum,” says Phelan, a self-described social entrepreneur. “What I hope is that conservationists figure out how to make this a new tool in their tool kit and that they use it cautiously, judiciously, appropriately, and with a lot of thought and a lot of input from ethicists, the public, and biologists.”

(Illustration: Donna Almendrala)
Karl Campbell meets me at IC’s headquarters across the street from Natural Bridges State Beach in Santa Cruz. He’s flown to the U.S. for a few days from the Galapagos Islands, where he’s spent the better part of two decades ridding the chain of volcanic islands off the coast of Ecuador of invasive mammals, which have taken a toll on endemic species like the Galapagos tortoise.
Cats, rabbits, donkeys, goats, pigs, rats, mice — Campbell has eradicated them all. Rodents, you might say, are his specialty. He’s led projects to eliminate rats and mice from 12 Galapagos islands. (He’s also, by the way, worked in California on San Nicolas Island, one of the Channel Islands, to boot out feral cats.) All that time spent in the field has made him a practical man looking for practical solutions.
He explains his current undertaking in the Galapagos to remove black rats and house mice from Floreana Island with rodenticides. It’s the hardest invasive mammal removal project he’s ever undertaken because, unlike in all the others, people inhabit Floreana. Small children would have to temporarily leave the island and livestock would have to be corralled, lest they ingest the poison, and all this has to be done with unanimous community support.
“But Floreana is nothing. Floreana has 140 people,” he says. “There are thousands of other islands we would love to be working on that are way bigger and way more complex than Floreana. And so if we’re challenged at this level to even be working on the simple end of the spectrum with these islands, then you know it’s a real long shot to be considering other places.”
In 2011, faced with the prospect of running out of islands where rodenticide is a feasible solution, he launched an effort at IC to look for alternatives that are socially acceptable, are easy to deploy, and hit the chosen species hard without collateral damage to other species.
“What really floated to the top of the list was these genomic tools. They fulfilled all those criteria we were looking at and possibly more,” he says. “And when we looked around and asked who’s working on this, there was no one.”
But the theoretical underpinnings were there. Techniques for creating gender distortion in a population, the so-called “daughterless” approach, had successfully skewed insect populations in laboratory tests and had even been found possible with lab mice. It was a big moment for IC to move into the domain of scientific innovation, and for Campbell the goal of the new genetic technology is complete eradication of a local population of invasives rather than a more limited form of control that would require re-visiting the same spots over and over for the foreseeable future, much like the work of pulling up French broom from the hillsides of Mount Tamalpais.
Once Campbell connected with the NC State team, it was only a few months before they co-hosted a workshop in Raleigh and laid out the issues. Top of the list was how a bioengineered mouse would spread its daughterless genes quickly through a wild population. The whole concept of autocidal genes seems to be so disadvantageous to a species as to fly in the face of evolutionary theory.
(Illustration: Donna Almendrala)
In evolution, many genes — the sections of DNA that are responsible for all of our traits — are passed along through generations and spread within populations because they presumably help a species in some way. While an individual has a 50/50 chance of inheriting a given version of a gene from either parent, those that increase an individual’s chance of survival and ability to reproduce are more likely to get passed along to the next generation. But scientists have also identified something called “selfish genetic elements,” nicknamed “selfish genes,” that advance their likelihood of inheritance regardless of how useful, or even harmful, they may be to the organism. These genes have evolved in a variety of ways and in countless organisms to beat the inheritance odds by as little as 50.001 percent to as much as 99 percent. They’re so ubiquitous as to be “a universal feature of life,” according to Austin Burt, the evolutionary geneticist who identified a means to exploit selfish genes in 2003.
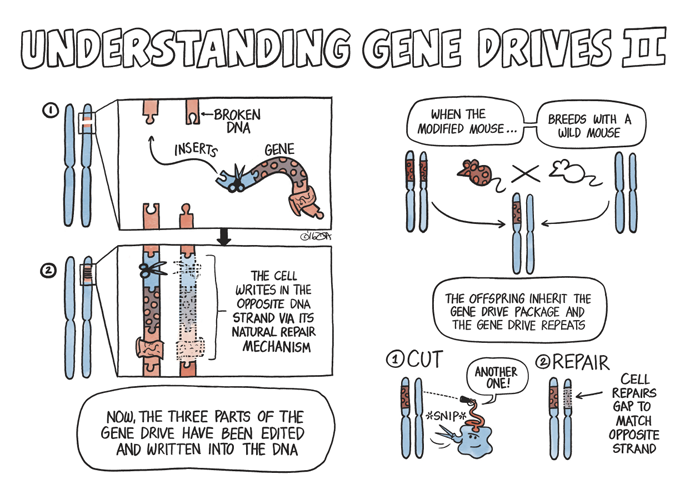
Scientists could, Burt found, artificially bind a gene with a desired trait to a selfish gene and then insert the package into the sex cells of an organism. Once they’re attached to each other, the selfish gene would effectively shuttle the desired gene into a population by increasing its probability of being inherited. The technique is called a gene drive — and is proving potentially useful in this burgeoning field of conservation genetics.
While gene drives occur naturally all the time, they vary in every species and can be difficult to identify. CRISPR-Cas9, a naturally occurring selfish gene found in bacteria, can function as a gene drive in other species — be it in a mosquito, mouse, oak tree, or human. Once placed in a cell’s nucleus, CRISPR-Cas9 will locate and snip out any specified section of the cell’s DNA, and the cell will repair the damage by copying CRISPR-Cas9, along with the desired gene, into the chromosome.
This is where the CRISPR-Cas9 system comes in handy. While gene drives occur naturally all the time, they vary in every species and can be difficult to identify. CRISPR-Cas9, a naturally occurring selfish gene found in bacteria, can function as a gene drive in other species — be it in a mosquito, mouse, oak tree, or human. Once placed in a cell’s nucleus, CRISPR-Cas9 will locate and snip out any specified section of the cell’s DNA, and the cell will repair the damage by copying CRISPR-Cas9, along with the desired gene, into the chromosome. A similar process will occur on the other chromosome, assuring two copies of the gene with the desired trait will be passed on to all offspring.
In the case of Mus musculus, there are classical genetic studies from the 1970s and ’80s of wild mice caught in Europe that possess a natural gene drive. “That’s where we started to explore,” David Threadgill says. “Could we use natural components present in mice for the conservation work?”
It was a tactical move to exploit a natural gene drive system for the project rather than CRISPR-Cas9, which would mean adding bacteria genes to the mouse genome to create a transgenic organism. Threadgill figures a natural gene drive is an easier concept to get past regulators and the public when the time comes for field tests with bioengineered mice. “This is already present in wild populations of mice, whereas CRISPR is artificial.” The natural gene drive system is probably a little less potent too, not a bad thing when it is introduced into the wild for the first time, he added.
It took a while, but Threadgill eventually tracked down a few specimens of these variant mice with the natural gene drive in freezers at the Pasteur Institute in Paris, France. “We basically recovered mice that had been frozen embryos and [they] now have a breeding population.” Threadgill’s lab is taking those house mice and pairing the natural gene drive system with a gene known as SRY that determines gender in mammals including house mice. By moving SRY off the male sex chromosome to the spot on the genome that contains the natural gene drive, Threadgill can make sure all offspring develop male reproductive organs, regardless of whether they have sex chromosomes that would normally result in the development of a female. “If SRY is connected to a gene drive system, then the vast majority of mice will inherit SRY and thus be male.”
Although such a daughterless gene drive system has never been built before in a mouse, progress has gone well over the last few years. As of April, Threadgill’s lab at Texas A&M was ready to pair the SRY gene with the natural gene drive system. “This is the last step of the process,” he says. He expects to produce the first fully assembled, bioengineered mouse by the end of this summer.

(Illustration: Donna Almendrala)
Are we ready for a world where whatever ails wild populations of species (or us) can be “fixed” with a tweak of the genes?
Though humans have long made an indelible mark on the genomes of wild species, breeding wolves into dogs and teosinte grass into corn, the precision and rapidity of today’s bioengineering methods raise innumerable possibilities and ethical questions. You could rightfully be ecstatic or terrified, or a bit of both, about the future.
What if bioengineered genes drift into another species, or out of a lab and into an unintended population? What happens if a bioengineered organism finds its way back to its native habitat — could that bring about the global eradication of the house mouse, or the mosquito? Or what if a rogue scientist, or a bioterrorist, decides to set loose a bioengineered organism, to hell with the consequences?
Even as scientists work on safeguards — like reverse gene drives that can overwrite genetic changes if something goes wrong, or immunization gene drives to inoculate non-target species from an altered gene — it’s clear that regulators and the public need to catch up with the science. A National Academy of Sciences review of gene drives, released in June, cautiously endorsed the technology but advised that more laboratory research and contained field trials be done before releasing a gene drive into the wild.
But beyond the white papers and scientific reports, the questions that biotechnology like CRISPR-Cas9 and gene drive systems raise in conservation hit at our philosophical relationship with the natural world. Given that we’ve already made a grand mess of every place on Earth, do we dare go to the deepest level of the genome, to the code of life? And what does it mean for a creature to be “wild” when it’s been bioengineered to do our bidding?
The Long Now Foundation’s offices at Fort Mason include a bar-cafe called The Interval, which has a steampunk aesthetic, all wood and metal, with displayed prototypes of the non-profit’s 10,000-year clock and a floor-to-ceiling library to jump-start civilization should it ever wink out, like the genetic code of an extinct species. Two taxidermied passenger pigeons on loan from a museum are positioned, lifelike, behind a glass case. Ryan Phelan meets me there and shows off the space.
She says she doesn’t see these new genetic tools as all that different from other longtime trends in conservation. “The truth is we manage wilderness a lot already,” she says, noting selective breeding programs, artificial insemination, relocating individuals, and other methods routinely used to bring back a struggling species.
“It’s the opposite of what synthetic biology wants to do, which is make new amazing things. In conservation it’s: ‘No, we don’t want to do great big amazing things. We want to do tiny, just-enough things and back the hell off.’ … All you’re trying to do in conservation is maintain an existing truth.”
Stewart Brand, the founder of Whole Earth Catalog, drops by our table and adds his two cents to the discussion of bioengineering the wild. “It’s the opposite of what synthetic biology wants to do, which is make new amazing things. In conservation it’s: ‘No, we don’t want to do great big amazing things. We want to do tiny, just-enough things and back the hell off.’ … All you’re trying to do in conservation is maintain an existing truth.”
Tinkering with a gene or two so that nature can re-balance itself may sound like minimal intervention to Brand and Phelan, but it makes others uncomfortable.
“My gut-level response is I don’t want people messing with nature any more than they have to,” says Doug Johnson, the executive director of the California Invasive Plant Council. Many restoration managers like Johnson — the people on the front lines of reclaiming habitat and battling invasive species — are taking a wait-and-see approach to bioengineering the natural world. Johnson says his group surveyed land managers in California and found that 90 percent of them use herbicides at least some of the time to control invasive plants. They want new tools at their disposal, but Johnson says a tool needs to be proven safe before it’s used.
In some cases, maybe even in the majority, advanced genomic tools might be too risky. Take, for example, the Invasive Spartina Project, an effort to remove non-native cordgrass from the San Francisco Bay using herbicides. Brian Ort, the geneticist working on the project, says a gene drive would be wholly inappropriate there given the close genetic relationship between the invasive Spartina alterniflora and the native variety experts are trying to restore. “We’re looking at two plants that easily form a hybrid. Anything you put into an alterniflora plant could easily get into the native population, and obviously we don’t want that.”
Yet then there’s the case of the American chestnut tree, a native to the Eastern Seaboard that’s been decimated by a fungal pathogen. By inserting a wheat gene into the tree’s genome, scientists at the State University of New York are creating blight-resistant trees that could be the first restored forest composed of genetically modified trees. Could a similar effort make California tan oaks resistant to Sudden Oak Death?
“I think it’s conceptually possible,” says UC Berkeley forest pathologist Matteo Garbelotto, who is trying to breed a SOD-resistant tan oak the old-fashioned way using genes found within the tan oak’s genetic repertoire. “I think the dividing line for me comes at using native genes versus non-native. If the process was solely based on genetic resources that are already available to the tan oak, I don’t have a big problem using that because I live day after day on the other side of the coin where all these trees are going to die. But if genes came from a different organism or tree species, I would start questioning the process. I’m not comfortable with that extreme level of genetic modification.”
So far, Karl Campbell says he doesn’t see many downsides to a bioengineered mouse, which in this case would remain genetically 100 percent mouse, though built in a lab. Worse, he says, would be inaction. “You can call it playing God, but the other part of it is, OK, cool, sit back and wait 30 years before you decide to get engaged. Meanwhile, you’re thousands of species short of where you were and, by the way, by the time you get to this it’s going to be tens of thousands of species.”
Though he feels confident, he knows the stakes are high to get it right. “Basically if you screw this up the first time, you will set this [type of effort] back three decades or more.” So the team is treading carefully, “go slow to go fast” is how he puts it. He’s initiating an independent panel that will look at potential pitfalls of releasing the mouse on a small island and then test out concerns. As far as picking an island for the first field tests, he says, the place will need a mature regulatory body that can give a gold standard review. And it will probably not be on the Farallones. “Thumb in the wind for social license? It’s likely not there.”
IC aims to start field tests in 2020. By then IC has to raise $6 million for the project, and Campbell knows he has to sell his story about islands. Ultimately, it’s an uplifting one. “I don’t often get back to the islands that we’ve removed invasive species on, but the ones I’ve gotten to are absolutely spectacular,” he says. Santiago Island, once free of goats and pigs, saw the immediate rebound of the nearly extinct Galapagos rail. “They were literally everywhere — ‘cheep, cheep, cheep’ — and just calling and going nuts. You read Darwin’s accounts of the island and you’re like, ‘OK, I can see it now.’” Rábida Island was cleared of rats in 2011, and two years later two species of land snail appeared that were last seen when the California Academy of Sciences collected them in 1905. “I find it super motivating, this work,” Campbell adds. “My wife asks, ‘When are you going to stop doing this?’ Well, when I’m dead.”
Back in Raleigh, John Godwin is getting to know the Farallones mouse really well. He sent a graduate student on a supply boat out to the islands to trap 14 live mice to start a colony in his lab. “So, what we have is Farallones mice breeding here in Raleigh,” he says. “We have them in a mouse barn.”
He explains that, because of the variation in mouse genomes (one supersize version of Mus musculus living on an island in the South Atlantic eats albatross chicks), he needs to run competition trials between the Farallones mouse and the European strain that’s slated to become the bioengineered mouse to assess its ability to successfully breed. “I jokingly say these male mice will have to have game. If they can’t compete with the local (Farallones) males in this population or if the females won’t breed with them successfully, this is not going to work.”
One way around that, Godwin says, would be to create a hybrid mouse with a high portion of the Farallones mouse genome and use it to carry the natural gene drive. That version of the mouse, “wild” enough to breed well, would be crossed with the bioengineered mouse (when it’s ready) and new fitness trials would commence — with the whole project moving over to a high-security laboratory, probably a Department of Agriculture facility in Colorado. “We want to be careful that they don’t escape and breed with mice living in the walls of the building or something. We’re never far from the house mouse in Western civilization.”
Godwin explains more lab trials with mice from a selected field trial location will eventually occur, but for now the Farallones mice are proxies for island mice worldwide: If all goes well with mice, who knows what’s next.
Not since his graduate school days at the University of Hawaii, where he studied the population ecology of coral reef fish, has Godwin been involved in a conservation project. “I feel like I’m back to dirty fingernails biology,” he says. “The motivation here is that we’re facing a severe biodiversity threat, and maybe there’s another way to approach this.”

||
This story originally appeared in Bay Nature magazine as “Re-Coding for Conservation” and is re-published here with permission.





