Millions of containers, thousands of ships, hundreds of scientists, 30 laws, 15 federal agencies, and we still can’t prevent the next foodborne illness outbreak.
By Kathryn Miles
(Illustration: Pacific Standard)
In an apocalyptic landscape, they wait. Trucks — dozens of them — parked outside places like Jim’s #2 Char-Broiled Burgers and Dreams Club, where you can see live nude girls, girls, girls and a special laser teaser on Tuesday nights. They hover around JJ Diesel and Capito’s Used Auto Parts. They idle on run-down side streets, next to overturned dumpsters and trashed shopping carts and the carcasses of dead dogs. It’s a no-man’s land, better suited for the set of a Mad Max movie than the staging grounds for America’s biggest import hub.
It’s also a landscape of necessity: the unseen backdrop to the 43 miles of congested waterfront and 7,500 glistening onshore acres that make up the Port of Los Angeles. Two million metric tons of food passed through the port last year, shipped by nearly 8,000 importers from over 100 countries — nations as far-flung as Bahrain, Mauritania, Cambodia, and Senegal. Thailand beat out China as the top importer at the port — but barely. Chile, Guatemala, and Vietnam round out the top five, with India close behind. And from these countries come every food imaginable (along with some you’ve probably never considered to be food): grapes and granola, cobra heads and desiccated camel, all destined for an American table.
But before it gets there, that food will make its way through a byzantine system of regulations and rules proctored by 15 federal agencies. Those idling 18-wheelers will shuttle most of it to a series of local warehouses, where it will sit while whirring computers and overworked inspectors decide if it warrants scrutiny. Some of it may travel to laboratories as far away as Pittsburgh and New Jersey to be tested. The trucks wait there too, while food scientists pulverize and distill it, looking for pathogens and poisons. Then it’s back to the warehouse, on to a distribution center, and, finally, to a grocery store near you — all part of a process to determine whether this food, which has already traveled thousands of miles and passed through untold numbers of hands, is safe to eat.
Making that determination is harder than ever. An estimated 48 million Americans become sick each year because of something they ate. Annually, over 3,000 die because of contaminated food. Both numbers are projected to rise in the coming decade, along with our reliance on imported food. And here’s the irony: A big reason for that increase is that we’ve developed healthier eating habits. On average, we eat 14 percent more fruits and vegetables than we did in 1970. We’re eating beet greens with bee pollen and drinking kale-and-date smoothies. And those foods — which is to say fresh foods — are the very hardest to police, particularly when they come from overseas.
The two biggest foodborne illness outbreaks of 2015 were caused by tainted produce (cilantro and cucumbers). The third was from deli items made at a natural food co-op in Idaho. Other large outbreaks were caused by tuna, pork, and salads.
“There’s a very slim chance potato chips or snack cakes or any other processed foods are going to make you sick,” says Michael Roberts, executive director of the University of California–Los Angeles’ Resnick Program for Food Law & Policy and author of Food Law in the United States. “The food that causes us the most problems is the food Michael Pollan tells us to eat, like meat and fresh produce.”

Most of this food falls under the purview of the Food and Drug Administration, which is responsible for regulating about 80 percent of what we eat. That includes products like fruits and vegetables, rice and grains, and dairy products. Unless, of course, you’re wondering if a pesticide used on one of those items is safe, in which case you’d have to ask the Environmental Protection Agency. The FDA has oversight of all fish and shellfish, except for catfish, which falls under a branch of the United States Department of Agriculture known as the Food Safety and Inspection Service. Nor does the FDA grade the quality of fish, since that’s the responsibility of the National Oceanic and Atmospheric Administration. Inspection of meat also falls to FSIS, unless that meat is considered wild or exotic, in which case it is the responsibility of the FDA (although FSIS may also inspect such meats). The FDA inspects shelled eggs and laying hens; responsibility for liquid, frozen, or dried egg products, along with meat birds, however, lies with FSIS. For foods with multiple ingredients, things get even more convoluted. Responsibility for the meat inside sausage falls to FSIS, but the casings must be inspected by the FDA. Foods like ice cream or television dinners may need to be inspected by both agencies at multiple stages. If the food is imported, Customs and Border Protection is responsible for screening the shipment upon arrival, though the FDA or USDA may need to inspect it as well. The standards, protocols, and guidelines at work here were developed in conjunction with the Centers for Disease Control and Prevention, which also bears responsibility for investigating outbreaks of foodborne illnesses. When such an outbreak occurs, the FDA, CDC, and FSIS convene what they call “a multiagency coordination group.”
Even the people inside this system agree: It’s a real headache. The Government Accountability Office believes the fragmented nature of the system results in inconsistent oversight, ineffective coordination, and inefficient use of resources. “It’s a situation that potentially places public health at risk,” says Steve Morris, director of Food Safety and Agriculture for the GAO.
To get inside this juggernaut, I had wanted to trace a single product — like a banana — as it moved through the process. I figured I’d start in Central America, where it was grown and processed, maybe hop a ride on a container ship, then follow the shipment though inspection and into the marketplace. That was before I learned the first rule of food safety: You do not talk about food safety. I made multiple requests to visit one of the FDA’s five foreign inspection offices, which dispatch inspectors to fields and factories in countries like Costa Rica and China. Each one was denied. I asked to visit the FDA’s operations at the Port of Los Angeles. This was also denied. Some of the only journalists granted access to these FDA facilities in recent years were a group of college students funded by an endowed program to advance young journalists. Their story, from 2011, which was picked up by publications including the Washington Post, described unbearable smells, understaffed facilities, and inspectors relying on their noses to determine if food was safe.
The staff at the FDA’s office of media affairs told me they didn’t think a visit to the port facility would add any insight to my story because, they said, finding “jaunty and colorful characters — is unlikely.” So I suggested visits to any of the 13 field labs, 20 district offices, or over 300 ports where FDA inspections take place. Those were rejected as well. In the end, months of requests and a final plea to visit any of the FDA’s food safety operations were all squarely denied. The CDC also denied my requests for interviews, referring me back to the FDA. Most third-party inspection labs — facilities that are under contract to test food for the FDA — did the same. Thus began my investigation into food safety: a secretive and labyrinthine process that seems to function with the expediency of a Rube Goldberg machine.
I was beginning to think I was going to have to trespass in order to report this story, but, eventually, the Customs and Border Protection agency broke the silence and invited me inside.
Beyond a line of concrete barricades in Carson, California, sits a sprawling 315,000-square-foot facility equipped with refrigerated warehouses, offices, labs, and inspection rooms. This is one of the first lines of defense for CBP, and a critical cog in the food inspection machine at the Port of Los Angeles. Each day, dozens of those idling trucks collect containers of food from the port and make the 12-mile drive to Carson, where they wait to gain approval to enter the facility. On the other side of a guard shack are 64 extra-large bay doors. There’s hardly ever a vacant one.
In an average year, the CBP staff at this facility — one of four “centralized examination stations” serving the ports of Los Angeles and Long Beach — will inspect about 3,300 shipping containers of food commodities sent from at least 15 different countries. Perishables like fruits and other temperature-dependent products are immediately sent to the refrigerated sections. Non-perishable items remain on the floor of the main warehouse, most stacked on pallets and intermingling with an array of non-food items — everything from guitars to flat-screen televisions. Even with all the cardboard and shuffling of goods in and out, the place is beyond immaculate.

A version of this story first appeared in the
of Pacific Standard.
.
CBP has its own mandates and laws to enforce, but it also works in close cooperation with the FDA and USDA, referring potential food safety issues along to the appropriate agency. On the day I visited, several staff were hovering over a bag of custard-filled cookies. Because that custard was made of eggs but the cookies themselves contained flour and sugar, the cookies seemingly fell under the purview of both the USDA and FDA, and they appeared to require further inspection. Someone somewhere must have known which agency needed to do what, and in what order. But in the meantime, a single sample pack of cookies sat alone in an enormous industrial refrigerator in an otherwise empty part of the warehouse. The remainder of the shipment might have still been on its container ship. More likely, it was sitting in a bonded warehouse somewhere nearby. To keep importers from releasing food into the market before it’s been cleared, the U.S. government requires them to pay a bond, which can be three times the value of the shipment. The bond is returned only after the food has been cleared by CBP and the FDA.
Customs and Border Protection is a relatively new agency — formed in the wake of 9/11 by combining the U.S. Customs Service and U.S. Border Patrol, as well as federal immigration and agricultural inspection services. It’s easy to spot who does what in Carson. The agriculture specialists wear blue CBP uniforms, making it clear you wouldn’t want to mess with them. They spend their days on warehouse floors looking for any insect or fungus that might prove a threat to American crops.
One hundred percent of all imported produce is inspected for agricultural pests before it can be released into the marketplace or even handed over to a distributor. Shipments are screened at the point of entry, and items selected for closer physical examination are immediately sent to a centralized examination station. A huge shipment of kiwis had just arrived from Italy on the day I visited. Inside a clean room, about a half dozen agriculture specialists were examining them, using magnifying glasses and high-test lights to spot tiny flea beetles the size of the period at the end of this sentence. These bugs are voracious leaf-eaters with a particular taste for crops like corn and lettuce. Dead ones aren’t a problem — it means the cold-storage shipping method has succeeded in killing any potential threats — but even a single living beetle might be enough to send the shipment back to its country of origin, prompt an enforced fumigation, or result in the shipment’s complete destruction.
My tour guide for this portion of the day, chief agriculture specialist Gabriel Padilla, wanted to underscore that the primary aim of his team is protecting domestic agriculture — not consumer safety. The import specialists at the Carson facility are an entirely different breed. They wear suits and wire-rimmed glasses and look like really, really smart accountants. Their desks are tidy, and they spend most of their days sitting at them. Instead of probing food samples, their job is one of paperwork forensics — a lot of which involves trying to ferret out whether any given food is what the importer says it is.
International trade agreements and domestic incentive programs have created a complex web for importers to negotiate, particularly with regards to anti-dumping laws intended to protect American producers from predatory pricing. A Chinese company, for instance, may be able to produce and import honey for just a fraction of what an American farmer needs to charge, so the Department of Commerce assesses tariffs on imports to protect domestic producers. Importers try to get around the extra fees either by routing the product through a country not affected by the tariffs or by passing off the product as something else (a lot of honey gets smuggled into this country labeled as apple juice). CBP investigators have also found boxes of seafood labeled as seaweed or garlic tucked into containers that are ostensibly carrying tube socks. While I was visiting, a Chinese produce importer tried to pass off a box said to be carrying vegetables but which actually contained electrocardiograph equipment. These sorts of violations are usually discovered via endless paper trails and the intuition of the specialists.
“It’s really hard to stay out of the weeds,” says supervisory import specialist Dave Shaw. “All you can do is start with the known, and hope that leads you to the unknown.” When Shaw’s forensic study of invoices and tariff forms raises a red flag, he sends uniformed CBP officers to inspect a shipment, usually at one of the bonded warehouses where it’s waiting to clear customs. If they see something suspicious, they email a photograph for him to review. If he thinks it’s fishy, he will recommend that the FDA inspect it further, in which case a sample will be sent to an FDA lab for testing. If the food is found in violation, the FDA will issue a notice of refusal, and the shipment will either be destroyed or returned.
In any given month, thousands of products fail to pass inspection by the FDA. During the month of my visit, items that had been refused by the FDA included “filthy” prunes from Afghanistan, Chinese cookies made with unsafe coloring, Indonesian bay leaves tainted with salmonella, and Ukrainian flax-seed oil containing traces of a prohibited pesticide. Nearly 2,000 products had been refused by the FDA in that month alone.
The FDA has oversight of drugs and medical devices as well as food, of course, and though it’s not immediately relevant to this story, I’d be remiss if I didn’t tell you that two of the items refused by the FDA that same month included devices for enhancing “external penile rigidity.” (I’d also be remiss if I didn’t tell you that these devices were rejected because they were mislabeled and did not include sufficient instructions for use.)
When the FDA finds a food sample that poses a serious threat to American consumers, Customs and Border Protection will order one of those rigs to deliver the entire shipment to an undisclosed location, where it will be demolished. I asked if I could visit that location, but CBP said no, citing security reasons. They did tell me that the sole purpose of this location is to “render indistinguishable” any food found in violation. CBP officers use a variety of destruction techniques, including incineration and pulverization. My favorite method for achieving the latter is a giant steamroller. (Their public affairs liaison does not love that I am telling you that.)
Right now, the FDA’s most reliable means of determining what foods pose the biggest risk is something called “prior notice,” which arose out of the Bioterrorism Act of 2002. The prior notice policy stipulates that all importers must notify the FDA of what food is coming into the country and when. It’s a narrow window: Food arriving by ship requires an eight-hour advance notice; food arriving by truck requires just two. The data that the importer provides is plugged into a computerized screening tool called PREDICT, which employs an algorithm to assign a percentile-risk assessment to every item of food entering the country. High-risk foods known to be susceptible to contamination or adulteration automatically get a higher number. So does food from countries or companies with a history of violations. Those cookies that had been detained by CBP were made in China. They could have gotten a high score because of their country of origin (China being, well, China), or because they contained eggs, a notorious carrier of multiple foodborne pathogens, including salmonella.
Products with high-risk percentile scores — or even just those that have received moderate scores — are flagged for review. The FDA also may issue import alerts for specific foods that have been found in violation of U.S. requirements. Food products on an import alert’s “red list” are automatically detained at the border. In order for that food to be cleared, the onus is on the importer to demonstrate its safety, which often involves employing an FDA-certified third-party lab like Microbac Laboratories.
Located in an unassuming industrial park just outside Pittsburgh, Microbac’s Warrendale, Pennsylvania, facility is housed in two labs — one for food chemistry, another for microbiology — separated in the middle by a large industrial kitchen. The kitchen is where chemists break food down into an analyzable form using any means necessary. Atop the steel countertops sit restaurant-grade mixers, food processors, and blenders. The drawers are filled with cleavers and heavy metal mallets.
Hesham Elgaali, the facility’s managing director, holds a Ph.D. in food microbiology from the University of Kentucky and served as Indiana’s supervisor of food and dairy microbiology for six years. These days, it’s not altogether uncommon for him to spend hours standing over a cheese grater.
“Food comes in every possible form: hard, soft, gummy, viscous,” he says. “Breaking it down can be really, really challenging.” The week before my visit, Elgaali toiled for the better part of a day trying to figure out how to render a shipment of synthetic bones for dogs into an extractable sample. He ended up driving to Home Depot and buying a sander. It worked.
In a storage room next to the kitchen, products detained by the FDA red list wait in plastic milk crates to be similarly digested. The contents of the storage room vary depending upon the time of year. On my visit, it was filled with gallon-sized Whirl-Pak sample bags of oregano and sesame seeds, two high-risk items commonly found to contain salmonella. Nearby, a cart containing 60 or so sauces produced by an Asian company awaited analysis. These two batches of food will make very different circuits through the lab. The sauces will hang out in the chemistry division, where they can be tested for everything from sodium content to whether or not they are, indeed, gluten-free. The herbs and seeds will undergo microbial testing and analysis using a sophisticated combination of enrichments and incubation to grow the bugs and DNA fingerprinting to identify their genetic code. The results will be sent to the FDA by way of a 300-page report documenting the specific strain of any microbe that might be isolated, along with the precise testing mechanisms used and the name of every analyst who touched the sample. The importer is spared all these details, receiving instead a short memo that says clear or contaminated. Either result will launch its own series of next steps, including either the release of the item to a distributor (more trucks) or the destruction of the shipment (more steamrollers). Most food gets released. A 2011 FDA briefing stated that, of the samples analyzed over the previous year, only 8 percent of the products receiving a 100 percent PREDICT score had been found in violation. The violation rate for products receiving a 50 percent PREDICT score, however, was almost as high. A more recent analysis by the GAO showed improvement in the correlation between PREDICT scores and violation rates. But the fact remains, our very best guess about which foods pose the biggest threat to human health is still just that: a guess.
Despite all the regulations and scientists and facilities involved, the most notable thing about this system is the fact that only about 2 percent of all imported food ever gets examined or tested by the FDA. The FDA knows this food inspection system is vulnerable. In fact, it’s been telling Congress as much for years. And the Government Accountability Office placed federal food safety oversight on its “high risk list” in 2007 (where it has stayed to this day), citing risks to the economy and public health.

The following year, over 300,000 Chinese infants and young children became sick after drinking milk tainted with melamine, a chemical that increases the nitrogen in milk and can mask the effects of dilution. Many of those children developed severe kidney damage. Six of them died. That same year, the Westland/Hallmark Meat Company, which supplied meat to American schools, initiated the largest meat recall in history after undercover video revealed its torturous treatment of “downer cows” — animals that are too sick or injured to move on their own. Congress responded to these and other food safety concerns by passing the Food Safety Modernization Act in December of 2010 — the first real reform to food safety laws in over 70 years.
On paper, the FSMA is an indisputably big deal. The FDA now has the authority to require what it calls “prevention-based controls across the food supply.” This includes requiring food processors and manufacturers to demonstrate compliance with protocols and to establish action plans in the event of an outbreak or violation. It also compels importers to provide certified verification that their food is safe. And, for the first time in U.S. history, the FDA can now demand mandatory recalls of any food product (prior to the FSMA’s passage, they could only politely ask).
Some key changes mandated by the FSMA are aimed at improving oversight and streamlining inspection processes by conducting more of them where food is produced, rather than when it is made available for consumption. The FSMA does this in part by putting food safety inspectors in foreign field offices and on the floors of processing plants, instead of just nosing through shipping containers and distribution centers. It is also supposed to close loopholes and help prevent microbe outbreaks like the ones at Chipotle this past year, or the cantaloupe tainted with listeria that left 33 people dead in 2011.
Randy Phebus, professor of food safety and defense at Kansas State University, is hopeful about the changes American consumers will see as a result of the FSMA. His involvement in food safety dates back to 1993, when over 700 people were infected with E. coli after eating burgers at Jack in the Box. Four kids died. Another 178 people were left with kidney and brain damage.
One part kitchen, two parts mad scientist’s lair, Phebus’ Manhattan, Kansas, lab is outfitted to conduct the very kinds of investigations that the FSMA calls for: testing food-handling procedures, rather than the food itself. Special freezers set at -80°C hold vials of salmonella, listeria, and Shiga-toxin-producing E. coli — some of the most pernicious bacteria responsible for foodborne illnesses. Next to the freezers sit utterly average-looking refrigerators, along with stoves and chemical wash stations. One day, Phebus will defrost a vial of E. coli and deliberately infect a batch of spinach with it, then send the spinach through the triple wash touted on the label to see if it really gets rid of the bug. The next day, he’ll mix some of the same strain of E. coli into ground beef and see what happens when you cook a burger rare (hint: Phebus eats his burgers well-done). He also stocks an inventory of surrogates — microbes that behave the same way as the nasty ones, but don’t have any negative effects on humans. Those get deliberately dispersed inside factories and food processing plants so that Phebus can see if the conditions there cause them to die or thrive.
“We’re never going to be in a position when we can test everything that comes off a processing floor, and we also can’t just sit back and hope that everything is right,” Phebus says. “The only hope is using science and engineering to validate the processes.”
Phebus may be optimistic about the recent changes, but some believe that the FSMA is taking too long to get off the ground. Part of the legislation included a congressional mandate that the FDA create safety standards within 18 months of the act’s approval. Without those regulations, there would be no means by which to implement the provisions of the act. After the deadline passed and no standards had been finalized, the Center for Food Safety, a national non-profit public interest and environmental advocacy organization, sued the FDA. The FDA led a motion to dismiss the complaint. The motion was denied, and a U.S. District Court ruled in favor of the Center for Food Safety, so the FDA led a motion to have the case re-considered. That, too, was denied, and so the FDA brought the case to the U.S. 9th Circuit Court of Appeals, which also ruled against the FDA. Eventually, in 2014, the agency consented to a court-approved agreement that compelled it to establish standards and dates by which they would be met. These rules were finalized in 2015 and 2016, and most will begin to take effect this fall. Some requirements, like the labeling of produce from small farms, won’t be fully in effect until 2020, presuming the rollout stays on schedule.
When the FSMA was passed, it also included an ambitious plan to investigate foreign food facilities, beginning with 600 international locations in 2011 and increasing that number each year to at least twice the total inspected the year before. The FDA has lagged behind. In 2015, it should have inspected 2,646 foreign food facilities, but only completed about half that. When questioned by a Government Accountability Office investigative team, FDA officials said that the agency had not met and was not planning to meet the FSMA mandate. According to the GAO report, FDA officials “questioned the usefulness of conducting the number of inspections mandated by FSMA.” That didn’t sit well with the GAO. The FDA cites cost as one reason the agency is not keeping pace with the FSMA mandate for foreign food facility inspections.

Jaydee Hanson, senior policy analyst for the Center for Food Safety, is not surprised by a lack of transparency and follow-through at the FDA. He points to the agency’s dual mission of food and drug regulation as a major problem. “The FDA is an organization almost entirely focused on drug research,” Hanson says, “and their culture reflects that. We believe Americans deserve an agency dedicated to food and food safety — and one not driven by the secrecy that pervades drug trials.”
To understand how this dual focus affects the agency, consider Domenic Veneziano, the recently retired director of the FDA’s Division of Import Operations. It was Veneziano who, in the wake of 9/11, designed the FDA’s first bioterrorism protocols and established the Prior Notice Center, which the FDA touts as its first around-the-clock operational office. And it’s his office (still lacking a director as this story goes to press) that works with the FDA’s many field offices to decide what food makes it into this country. Yet last year, he spent months in the national spotlight not because of any particular issue or incident regarding food safety, but because it was also his job to prevent states like Arizona and Ohio from obtaining lethal injection drugs from overseas when domestic supplies ran low.
To an outside observer, the scales at the FDA do appear to be tilted toward the drug side of things. There is a marked lack of expertise in food science and environmental health among the agency’s leadership. The current commissioner of food and drugs at the FDA, Robert Califf, is a cardiologist who has a deep background in drug trials. His chief scientist is a medical researcher with experience in biodefense and infectious disease. Of the administration’s five deputy commissioners, only one is responsible for food oversight — a position that was established in 2010, and one that oversees all veterinary concerns as well.
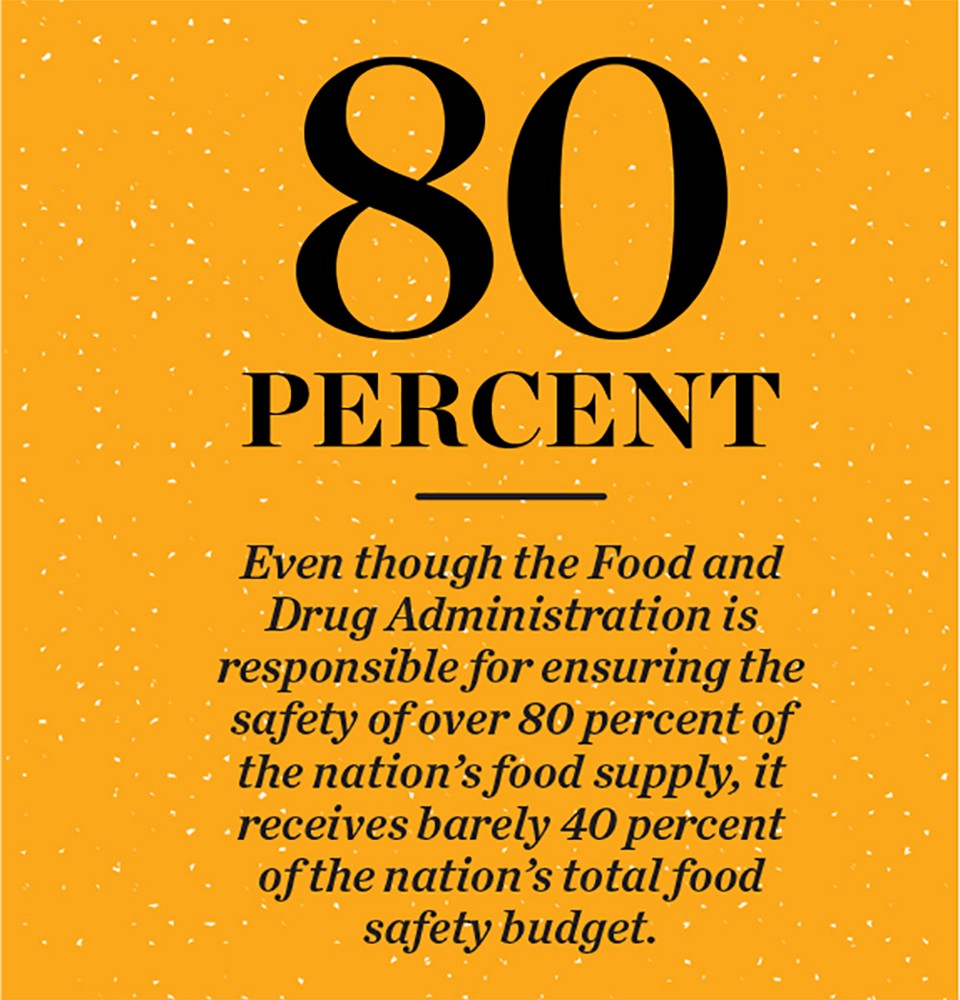
Funding is another issue. A recent white paper published by the Congressional Research Service reveals a long history of disproportionate funding between the FDA and the inspection arm of the USDA. Even though the FDA is responsible for ensuring the safety of over 80 percent of the nation’s food supply, it receives barely 40 percent of the nation’s total food safety budget — a discrepancy born out in staffing levels as well. For years, the USDA has had over twice the dedicated food safety and security staff as the FDA. Staffing issues are particularly severe at the FDA’s overseas branches, where visa issues and international agreements have led to partially filled offices and empty desks. And since the passage of the FSMA, lack of funding has become an even bigger problem for the FDA. When the FSMA was first enacted, the Congressional Budget Office estimated that the FDA would require a funding increase on the order of $583 million through fiscal year 2015 to properly implement it, but increases to the FDA budget during that time fell $276 million short of the estimate, which amounts to nearly half.
Michael Roberts of UCLA’s Resnick Program for Food Law & Policy thinks changing our perception of food might be the only viable solution. “You can double the FDA’s budget tomorrow and they still won’t have the resources they need to ensure the integrity of our food,” he says. “It’s about risk and degree. How pure do you want your food to be? How much are you willing to pay to make it safe? In the end, as hazards increase, it’s up to us to decide what we are willing to tolerate.”
Meanwhile, the food keeps coming. Each month, 100 or so container ships nose into the Port of Los Angeles, carrying hundreds of thousands of shipping containers, 20,000 of which are carrying — or said to be carrying — food. And so the truckers keep schlepping, the inspectors keep inspecting, the scientists keep sampling, while the CDC stands at the ready with its multi-agency protocol for responding to the next foodborne illness outbreak. That there will be one is a statistical certainty. The questions are: From which food? In what way is it compromised? And how many people will get sick or die as a result?




