Nestled on the narrow neck of a rocky peninsula that juts into the Pacific Ocean, the Seto Marine Laboratory is one of Japan’s oldest facilities for studying the abundant fish, marine invertebrates and seaweeds that have sustained people here for centuries. These days, the resort hotels that line the coastline of Shirahama — the name means “white beach” — are a far more important lifeline for the region’s economy than fishing. But in the laboratory, amid a welter of bubbling tanks and clattering pumps, a marine biologist named Yoshihisa Shirayama and his staff and student researchers are trying to understand how aquatic creatures adapt to a habitat in rapid flux. To that end, he and his colleagues have built an infrastructure that mirrors a changing ocean; with a few swipes across a touch-screen control pad, he can adjust the concentration of carbon dioxide in tanks that hold sea urchin larvae.
An urchin larva looks like a delta-wing fighter plane, albeit on a microscopic scale. It’s difficult to keep these creatures alive for long in the lab, but for a few days, as they begin their growth, they are splendid barometers of their environment. They need calcium carbonate, dissolved in seawater, to begin growing their hard shells. The amount of carbonate in water is a direct function of the concentration of carbon dioxide; as the oceanic CO2 level increases, the water becomes more acidic, and structures made of calcium carbonate begin to dissolve. And the carbon dioxide level, in the open ocean, directly mirrors that of the air.
In This Issue
Do bacteria think? Is Facebook a medical record? Can we reform welfare reform? Check out those stories, our cover story on dealing with climate change through ocean carbon sequestration, and much more in the November-December 2010 issue of
Miller-McCune magazine.

Since the beginning of the Industrial Revolution, the level of carbon dioxide in the atmosphere, and hence in the ocean’s surface waters, has increased by almost 30 percent, from about 280 parts per million to today’s level of about 390 ppm. In response, those surface waters have become steadily more acidic.
With its variety of tanks, the Seto lab allows Shirayama and his colleagues to precisely adjust living conditions for the sea urchin larvae. Some live at 280 ppm, some at 390. Others live at levels that the real ocean may experience in the not-so-distant future, assuming that human beings continue to burn fossil fuels and produce greenhouse gas emissions.
The equipment is complex, much of the work preliminary, its implications not ironclad. But so far, the results show a clear trend: As the level of carbon dioxide in the water increases, the sharp points of the larval triangles become less pronounced. By the time the level reaches 2,000 ppm — a concentration that, barring major changes in how we get our energy, the oceans may reach sometime between 2100 and 2200 — the juvenile animals are significantly shorter, more blunt-pointed. They become space shuttles rather than F-15s. They survive, but perhaps only because in the lab’s tanks there’s nothing to eat them. Such drastic changes don’t bode well for the sea urchins or for the other animals that share their living space.
The Seto lab is one of several worldwide that are pioneering the incipient field of research on ocean acidification, an effect of greenhouse gas emissions that may rival climate change itself in the impact it will have on global ecosystems and human economies. But it is also a place at the forefront of what is likely to become a major debate about global change: Namely, to what extent should people alter the oceans in an effort to deal with climate change? And Shirayama is in an unlikely position for a marine biologist. He finds himself advocating for a system that would sequester carbon dioxide deep in the ocean, a process that could blunt the worst impacts of climate change but might also change the ecology of the sea in dramatic and unpredictable ways.
You can’t go very far in asking Japanese scientists about carbon sequestration before being asked whether you’ve talked to Takashi Ohsumi, a puckish engineer with a sardonic laugh who is one of the experts in the field. He works, in semi-retirement, for the Central Research Institute of the Electric

Power Industry, or CRIEPI. In a stark conference room on the institute’s campus just outside Tokyo, he can spend hours giving a comprehensive overview of the country’s involvement with ocean carbon sequestration.
A brief outline goes something like this: In the late 1980s, faced with growing demand for electricity but intense opposition to new nuclear power plants, the country’s electric industry began considering the construction of plants that would burn coal imported from overseas. Officials already knew that the emissions from fossil fuels were likely to contribute to global warming, so they began investigating how to capture and sequester carbon dioxide.
The idea of deliberately depositing carbon in the oceans as a means of dealing with fossil fuel emissions did not arise in Japan — it was proposed by North American and European scientists in the 1970s. But as Ohsumi recounts, it was an idea ideally suited to Japan, a heavily industrialized island where volcanic geology doesn’t provide many opportunities for depositing carbon under its land area.
Using both at-sea tests and high-pressure tanks that mimic deep-ocean conditions, he and his colleagues began investigating how carbon dioxide behaves when deliberately deposited in seawater. In shallow water, the effect is much as you’d expect: Drop in a block of dry ice, and it bubbles and dissolves like the fizz in a carbonated drink, contributing its carbon dioxide to the atmosphere as much as to the water. But in deep water — say at about 1,500 meters — the result is different. Because of the increased pressure, the CO2 dissolves into the seawater and remains more or less at the same level beneath the surface. If the water is deep enough — more than about 3,000 meters — the carbon dioxide becomes a liquid denser than seawater and sinks to the bottom, where it pools and remains.
The oceans are already absorbing from the air something like a million tons of carbon dioxide an hour. In other words, people are already injecting large quantities of the gas into all the world’s shallow waters, causing the sorts of acidification problems that are under investigation at the Seto marine laboratory. But ocean waters mix slowly. Carbon dioxide placed at a depth of about 1,500 meters, Ohsumi came to believe, might take several hundred years to migrate into surface waters and then into the atmosphere. If it were placed in depressions at extreme depths — say of 3,000 meters or more — most of it would likely remain in place for thousands of years. Though neither tactic would represent a permanent solution to the problem of greenhouse gas emissions, either would buy time during which mankind could develop alternative energy sources.
By the mid-1990s, researchers from several countries, including Japan, Norway and the U.S., were investigating how ocean carbon sequestration might work in practice, and what its impacts might be. Would it work better to deposit the CO2 through a fixed underwater pipeline or from a moving ship? What would be the effect on fish or on organisms that live on the seafloor? How would deep-ocean currents or turbulence affect the length of time that carbon dioxide remains separate from surface waters? What about the formation of hydrates — ice-like lattices of interwoven carbon dioxide and water molecules that could keep liquid carbon dioxide sequestered from seawater but that also might clog the pipes used to deposit it?
By the turn of the century, scientists were ready to conduct experiments in the ocean. An international team proposed a site off the Big Island of Hawaii, where a deep-ocean pipeline was already in place. The sequestration plan seemed simple: Connect a smaller pipeline to the existing one, pipe about 50 tons of liquid carbon dioxide down it to a depth of 800 meters and see what happens.
As soon as word of the experiment reached the press, though, opposition erupted. Residents, fishermen and Native Hawaiian groups vigorously protested the project — and some protested the idea of sequestration itself, claiming that it would harm sea life or suck away money that would be better spent on developing alternative energy sources. Though the Hawaii Legislature and U.S. Department of Energy ultimately threw their support behind the scientists, the controversy caused the project’s proponents to withdraw.
Instead, they chose an alternate site off the coast of Norway and slimmed the size of the carbon dioxide releases to only about 5 tons. The project had a green light from the national government — until the Greenpeace ship Rainbow Warrior showed up in Oslo in the summer of 2002 to protest the experiment. “The project,” a Greenpeace press release claimed, “is not about better scientific understanding — it’s about vested interests attempting to ensure that the fossil fuel industry has a secure future. Research into ocean dumping is taking money and attention away from the real solutions to climate change — phasing out the fossil fuels, which release greenhouse gases, and replacing them with renewable energy such as solar and wind power.”
“Greenpeace propaganda claimed that mad scientists want to put CO2 into the sea” is how Ohsumi sums up the environmental group’s stance. Faced with this unexpected opposition, Norway’s minister of the environment abruptly withdrew the project permit.
Ohsumi, like many of his colleagues, was demoralized. Three months later, some of them met in Regina, Saskatchewan, to work on drafting a summary on ocean sequestration prospects and research for the next report from the Intergovernmental Panel on Climate Change. Such a chapter was included in the next report, issued in 2006. But by then scientists and policymakers were moving toward a new idea: placing the carbon dioxide underground, rather than in the ocean. Ocean sequestration seemed, so to speak, dead in the water.
“International cooperation,” Ohsumi says, “is like this,” as he makes his two hands take on the form of a slithering snake.
But the idea of carbon sequestration itself — often known as CCS, for carbon capture and sequestration — did not go away, in Japan or elsewhere. As the difficulties of making meaningful cuts in fossil fuel emissions become more and more apparent, many climatologists, engineers and policymakers are increasingly enthusiastic about — or at least resigned to — the idea of squirreling away carbon dioxide in places where it will remain safely separated from the atmosphere.
“More climate protection is possible with CCS than without it,” says Bjorn Utgard, energy policy adviser at the Bellona Foundation, a Norwegian nongovernmental organization that is cautiously supportive of sequestration. He believes that human beings will continue to rely on energy from fossil fuels for decades, and that sequestration is needed to mitigate the damage from their emissions. “If you do CCS on the biggest sources,” he says, “you don’t really need to have that many hundreds or thousands of projects before you really have an effect.”
Norway is a poster child for sequestration. It is a major producer of oil and gas, thanks to the extensive deposits beneath the North Sea. The methane in natural gas is typically found together with naturally occurring carbon dioxide, which producers have historically separated and vented into the atmosphere. Natural gas extracted from the Norwegian part of the North Sea contains about 9 percent carbon dioxide. In 1991, the Norwegian Legislature imposed a tax on the release of carbon dioxide through oil and gas production. With a stroke, it became cheaper to bury that CO2 than to release it, and since 1996, the national petroleum company Statoil has been doing exactly that, compressing and injecting about a million tons of carbon dioxide a year into a saline aquifer 800 meters below the bottom of the North Sea.
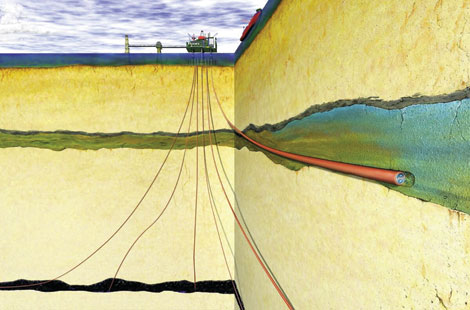
If you want carbon dioxide to stay put underfoot, you look for places where underground strata sit atop one another, like the parts of a complex layer cake. In the North Sea, for example, Statoil injects its carbon dioxide into a layer of dense sand mixed with saltwater. The carbon dioxide is kept from escaping its geological tomb by 800 meters of rock above the sand. Many places worldwide have this layered geological structure, but Japan, with its fractured igneous rocks and high potential for earthquakes, is short on such storage sites.
Engineers were able to find one place where they could do a test. Outside the city of Nagaoka, near the Japan Sea coast, a company named INPEX had been extracting natural gas from a small field formed in igneous rocks. That methane had stayed put there for millions of years suggested that carbon dioxide could, too. It didn’t hurt that local communities were accustomed to the sight of natural gas wells in the midst of fertile fields said to produce Japan’s best rice.
Beginning in the summer of 2003, engineers each day sent between 20 and 40 tons of liquefied CO2 down a 1,100-meter-deep well into a zone of gray-greenish tuff saturated with groundwater. Three monitoring wells that were drilled nearby allowed the engineers to follow how the carbon dioxide moved.
After 18 months, more than 10,000 tons of CO2 had been injected. This was, by almost any standard, a trivial amount; during the same period, INPEX separated about a hundred thousand tons of carbon dioxide from the methane it was mining from the Nagaoka gas field and vented it as waste into the atmosphere. But the CO2 has been meticulously monitored since 2005. The results have been encouraging; the carbon dioxide has largely stayed put — even during the magnitude 6.8 Chuetsu earthquake in 2004.
“CO2 is not so mobile — it stays around the injection well,” says Kozo Sato, an underground reservoir engineer at the University of Tokyo who has been closely involved in studies of the Nagaoka site. “It’s less mobile than we imagined before we performed the experiment.”
Those rosy results, though, don’t mean that carbon storage on land is going to take hold in Japan. Good sites simply don’t exist on land, and even if they did, it’s likely that local opposition would derail or slow projects. After the Chuetsu earthquake, some area residents were quick to accuse the injection project of causing the tremors — even though the epicenter was about 12 miles away from the injection site. Opposition to carbon burial under land has delayed or stopped projects in Ohio, the Netherlands and Germany, resulting in the coining of a new phrase: NUMBY, for “not under my backyard.”
Even so, research into carbon sequestration will continue; many believe that the enormous inertia behind the fossil fuel economy demands it. At the G8 summit held in Japan in July 2008, world leaders pledged to support carbon sequestration as a strategy for mitigating climate change by underwriting as many as 20 demonstration projects. Through its Ministry of Economy, Trade, and Industry, Japan will underwrite one of them. The plant’s construction and operation will be carried out by Japan CCS, a consortium of about three dozen industrial corporations in Japan, including utilities and oil companies.
METI and Japan CCS are examining two potential sites, one in a depleted natural gas field off the east coast of Honshu near the city of Iwaki and one just off the coast of the northern island of Hokkaido. The goal is to test sequestration as a complete process: 100,000 tons of carbon dioxide a year would be captured from either a power plant or refineries, then piped offshore and injected deep beneath the ocean floor.
“Technically, we do not have much concern,” says Yoshio Hirama, coordinator/general manager of Japan CCS. “We have transportation technology, we have gas pipelines, and we can convert that pipeline to a CO2 pipeline, taking into account the difference in gas. And we have technology to inject gas into underground reservoirs, but this time it’s the CO2 we’re going to inject.”
Still, carbon capture and sequestration is likely to work on a large scale only if the cost of capturing carbon dioxide from a power plant or any other industrial source can be significantly reduced. At a coal-fired power plant, operating the technology to capture waste carbon dioxide can consume anywhere from 15 to 40 percent of the plant’s total energy output. In Japan, officials calculate the cost at about $40 per ton of carbon captured — which will readily translate into higher electric bills. A number of researchers are trying to find cheaper chemical means of capturing waste CO2, but the technical challenges remain formidable.
So do the sociopolitical ones. In Japan, fishermen’s associations have long wielded significant influence on the industrial development of coastal areas. Representatives of those associations in the Iwaki area refused to be interviewed for this story, citing the delicate nature of ongoing negotiations. But the head of a fishermen’s association at Teradomari, the coastal location nearest the Nagaoka experimental site, was happy to talk. Kazuya Kaneda has been fishing for 48 years, and he makes no bones about his opposition to any burial of CO2 beneath the seabed.
“Because of the earthquakes,” he says, “we worry about putting CO2 under the seabed. Nobody can guarantee that such a project would not cause an earthquake. This kind of project would never have public support here because people would worry too much about leakage.”
DUMPING OF wastes at sea is regulated by the 1972 London Convention. In 2006, following the debacle of the proposed ocean sequestration experiments in Hawaii and Norway, the signatories amended the convention to allow disposal of carbon dioxide under the seabed, but not in ocean water itself. But advocates of sequestration in — rather than under — the sea insist that their idea is not dead.
There are three principal reasons why ocean sequestration may come to appear attractive in comparison to geological storage. The first is the potential opposition to geological sequestration epitomized by fishermen’s associations. Any particular tract of land, whether onshore or offshore, is going to have local values attached to it — and local concerns, well founded or not, about whether injected carbon dioxide could return to the surface. Though no one has proven any connection between carbon dioxide injection and earthquakes, scientists point out that it’s practically impossible to prove that a particular sequestration project won’t cause tremors, especially in a country as seismically active as Japan. On top of this are liability issues — who, or what, would be responsible if carbon dioxide were to leak from a sequestration site?
These challenges point up another, cost. Under the London Convention, wastes injected into the seabed are to be monitored indefinitely. Any company or country doing carbon dioxide injection would need to bear the costs not only of drilling injection wells, but of monitoring them. These additional costs might well be large enough to make an undersea CCS project impractical.
In the abstract, disposing of CO2 in the ocean, rather than under the seabottom, seems to face fewer political problems. There’s no particular local constituency for the deep ocean. If carbon dioxide is disposed of in water, a mile or more under the ocean surface, there’s no way for it to be released catastrophically into the atmosphere. If it’s dissolved in the mid-ocean, there isn’t even any way for it to be monitored over time; it will eventually simply disperse.
“Of course, for us, ocean sequestration is like killing two birds with one stone: technologically easier, and the cost is lower,” says an official at METI. “However, even if it’s a small amount of CO2, it’s very hard to get acceptance just to the idea of dissolving CO2 in the ocean. We’re not rushing to pursue this. But we haven’t given up the idea of pursuing ocean sequestration. Our idea is to first focus on developing technology for subsea carbon sequestration and continue to pursue the technological aspect of ocean sequestration at the same time.
“When the time is right, maybe we will openly ask for permission for our position of wanting to pursue ocean sequestration.”
There is a huge drawback to sequestration of carbon dioxide in ocean water: ocean acidification, which Yoshihisa Shirayama is studying at the Seto Marine Laboratory. If carbon dioxide is deliberately placed in the ocean, at whatever depth, it will ultimately reach surface waters and contribute to their acidification. The results are intimately familiar to Shirayama. The larvae of sea urchins and other marine organisms with external skeletons will grow differently. Adults will grow less and have more trouble surviving. Shellfish will be unable to develop shells. Corals will no longer build reefs. Even fish, which are comparatively robust and can withstand high levels of dissolved carbon dioxide, may be affected: New research has shown that fish larvae exposed to high levels of dissolved CO2 lose their fear of predators. The results of all these ecological changes on economies that rely on the sea will be enormous.
So why would a marine biologist countenance the idea of ocean sequestration? The answer lies in a long-term — some might say pessimistic — view of humanity’s probable response to climate change. Like many advocates of ocean sequestration, Shirayama is not optimistic about efforts to rein in fossil fuel emissions. Without major mitigation efforts, he believes, carbon dioxide concentrations in the atmosphere — and hence in the ocean’s surface waters — might reach as high as 2,000 parts per million by sometime between 2100 and 2200, or some five times the current level.

Imagine that he’s right — and that carbon dioxide levels stabilized there. As shallow ocean waters slowly mix with deeper waters, atmospheric levels of carbon dioxide would begin to decline; over millennia, some of the carbon dioxide in deep-ocean waters would become bound up with sediments. The 2,000 ppm level would represent a short-term peak that would cause great ecological and economic damage, but over a few centuries, CO2 levels would decline again.
Now imagine that carbon emissions remain the same — but humans decide to extract a lot of CO2 from industrial smokestacks and pump it into the deep ocean. Of course the carbon dioxide will re-emerge — but advocates say that it would do so over hundreds or even thousands of years, so the atmosphere and surface waters would never reach that damaging peak concentration of 2,000 ppm.
Ocean sequestration, in other words, would speed up the otherwise centuries- or millennia-long process of establishing equilibrium between the atmosphere and the entire ocean. It would not decrease acidification of the entire ocean, but it might limit acidification in the surface waters that are of greatest economic interest to people. In a world of limited resources, it may represent the most efficient way to store a lot of carbon quickly.
Shirayama says the decision on whether to pursue ocean sequestration revolves around a tradeoff. “If you do not do it, the surface ocean may be impacted,” he says. “If we do it, the impact on the deep ocean might be larger.”
So ocean sequestration may be a bad idea that will cause untold harm to deep-ocean ecosystems we barely understand — but doing it may also represent a better alternative than doing nothing. It’s like the amputation of a badly wounded leg: a terrible prospect, unless it’s the only way to save a life.
Ralph Keeling, director of carbon dioxide studies at the Scripps Institution of Oceanography, says it is “extremely unlikely” that it’s worse to put carbon dioxide into the ocean than, uncontrolled, into the atmosphere. He authored a recent paper arguing that in a climate change world, advocates for every ecosystem will have to give something up to protect the geochemical stability of the entire globe.
“It’s clearly not the best option,” he says. “But there is not a silver bullet.”
The best option would be to greatly reduce carbon dioxide emissions. And some critics argue that sequestration research is a distraction from that solution. “CCS is complete rubbish, both because of its technology as well as political context,” Tetsunari Iida, director of the Institute for Sustainable Energy Policies, a Japanese NGO, wrote in an e-mail. “History tells us that technological fix solutions always fail.”
Do humans have the collective willpower to wean themselves from fossil fuels? However that question is ultimately answered, ocean sequestration of carbon dioxide is likely to be pursued somewhere, somehow. It likely will re-emerge as a real policy alternative, if not in this decade, then maybe in the next; if not after the next powerful hurricane or deadly heat wave, then after whatever subsequent disaster best epitomizes mankind’s energy-climate crisis; if not in the U.S., then in Japan, or Korea, or Taiwan — all heavily industrialized nations with large carbon footprints, ocean frontage and few good options for other means of sequestration.
“Scientific society in Japan has not given up on ocean sequestration,” Shirayama says. “Maybe in 10 or 15 years — in the not-very-far future — we need to think about it. Or we need to change our energy economy so that we are not using oil. Maybe that would be the best solution.”





