Headaches. Insomnia. Anxiety. American medicine cabinets are packed with remedies for these common maladies. And up to 40 percent of them are manufactured overseas (along with 80 percent of active ingredients for pharmaceuticals). But a recent report by the U.S. Government Accountability Office estimated that in fiscal year 2009, the U.S. Food and Drug Administration visited just 11 percent of the 3,765 foreign factories it is responsible for inspecting — compared to 40 percent of domestic factories. In 2008, the GAO found that the FDA took two to five years to follow up with foreign plants it cited for safety issues — if it followed up at all.
The Jan-Feb 2012
Miller-McCune
This article appears in our Jan-Feb 2012 issue under the title “Pills Without Borders.” To see a schedule of when more articles from this issue will appear on Miller-McCune.com, please visit the
Jan-Feb 2012 magazine page.

In 2008, 30 products made by a single Indian company were banned by the FDA, and a tainted batch of the blood thinner heparin from one of many hundreds of Chinese pharmaceutical plants was linked to 81 U.S. deaths.
The good news is, the low rate of inspection should soon change: under an agreement reached in August between the generic drug industry and the FDA (expected to win congressional approval in 2012) the generic drug companies would pay $299 million in annual fees to help the FDA inspect their overseas operations. Inspections would happen once every two years, the same rate as at U.S. facilities.
And yet, over-the-counter drugs remain outside the scope of the new agreement. (Nearly all the aspirin and vitamin C consumed in the U.S. is made in Chinese plants that never see an inspector.)
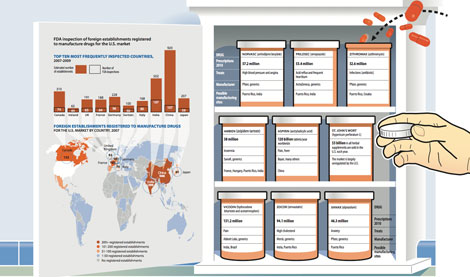
Here is the “Pills Without Borders” graphic as it appears among the “Medicine on the Front Lines” reports in the January-February 2012 issue of Miller-McCune magazine.
Sign up for the free Miller-McCune.com e-newsletter.
“Like” Miller-McCune on Facebook.
Follow Miller-McCune on Twitter.
Add Miller-McCune.com news to your site.





