Let’s say, just for the fun of it, that rats engage in speed dating, and we have a hidden camera. Rat A — let’s call her Betsy — is ready and willing to select a mate from a pool of males who are milling around in a separate room, downing too many cocktails. One by one, the male rats visit Betsy as she sits, nervous and inquisitive, at a little round table, toying with her bar napkin.
Rats routinely identify each other and maintain relationships via a lot of sniffing, nosing and general snorfling, and when they’re in the mood for love, they do it even more. Alas, all the snorfling in the world will not reveal Betsy’s Mr. Right tonight. Many of the potential mates who come her way just seem wrong somehow, and Betsy rejects them.
Is Betsy merely an overly picky girl destined for spinsterhood? No. Betsy is just observant — observant enough to preserve her species. You see, Betsy is a Sprague Dawley rat, a variety commonly used in biological experiments and the very kind used by Andrea Gore and David Crews of the University of Texas, Austin and Michael Skinner of Washington State University, Pullman, who have found something amazing and scary in a mate-selection experiment very similar to Betsy’s speed-dating scenario.
In the experiment, female Sprague Dawley rats were exposed to potential male suitors, some of which were descended from one great-great-grandmother rat who had been given a high dose of the fungicide vinclozolin when she was pregnant. Vinclozolin, used worldwide on a variety of agricultural products including wine grapes, is banned in Scandinavian countries, but the EPA continues to allow its use in the United States on certain crops.
Earlier experiments showed that male offspring of mothers exposed to vinclozolin were very likely to be sterile or produce sperm with impaired mobility and to develop prostate cancer, kidney disease and immune system problems as adults. Amazingly, normal females like Betsy were able to divine, probably by detecting and interpreting pheromones secreted by the males, which would-be suitors descended from that matriarch. The females spurned each and every one of them.
The “speed-dating” study — “Transgenerational Epigenetic Imprints on Mate Preference,” published in the Proceedings of the National Academy of Sciences in April 2007 — showed that the diseases persisted in the male rats for four generations. (The rats were bred young, before the diseases and the males’ reproductive problems manifested themselves.) Yet the susceptibility to disease was not because the rats’ genes had been damaged by the fungicide. Rather, it was an epigenetic phenomenon. That is, the fungicide had apparently affected the complex chemical processes required to package and activate or deactivate genes.
The studies by Gore, Crews, Skinner and their colleagues sit at the intersection of several major developments in biology. The vinclozolin acted as an endocrine disrupter, affecting male reproductive fitness. The diseases lying in wait for the adult male rats were a manifestation of the Barker hypothesis, which posits that events in very early development can result in strong susceptibility to adult disease. And the inherited disease susceptibilities are governed by the rules of epigenetics.
These paradigms were glimpsed years ago but blossomed in the last couple of decades, when high-throughput lab techniques to analyze genes and proteins became widely available. This scientific convergence is pushing seismic ripples throughout biology by remodeling long-standing assumptions about genetics. For a century after Gregor Mendel’s death, inheritable traits were thought to be determined by genes, the DNA elements in the nucleus of a cell, which could be affected by the environment only in limited ways.
Epigenetics, however, focuses on the chemical scaffolding that supports and packages DNA and activates or turns it off partially or completely. Because this scaffolding is susceptible to environmental influences, researchers now see it as central to both frightening and hopeful prospects for human health: It may be the avenue for a fearsome array of serious conditions — from cancer to obesity — that may be caused by exposure to only minuscule amounts of chemicals and that may be passed to offspring. At the same time, epigenetics holds out promise for preventing and treating those very diseases with relatively simple changes in nourishment and lifestyle.
DNA Dogma
Since its double helix was first described in 1953, deoxyribonucleic acid, or DNA, has been a monomania among both geneticists and a hefty chunk of molecular biologists, especially those interested in the development of animals, plants and other organisms. Ecologists and people who study individual populations, meanwhile, have focused on reproductive fitness and genes-versus-environment questions.
For all of them, though, Mendelian genetics was the governing dogma. The genotype — or the entire set of genes contained in the spiral of DNA inside every cell of an organism, half contributed by each parent — produces the phenotype, or the actual organism that encounters the environment. It was a one-way street: No environmental influence on the phenotype could change the genotype. Chopping off a mouse’s tail did not produce a line of tailless mice, no matter how many times the tails were chopped. Only those random mutations in what’s called the “germline” or eggs and sperm, probably caused by the occasional hit by a cosmic ray or a misalignment or transposition of a few segments of DNA during replication, could change an organism’s genetic legacy. Most of those random changes are either neutral or harmful, but once in a while you get an adaptive mutation that is beneficial to the organism, enhances its reproductive luck and produces the natural selection that is the basis for evolution.
Some scientists have long suspected that the DNA dogma was too limiting, however, and that the rate of genetic change was too slow to fully account for the evolution of species. “There’s always been a time problem in thinking about how evolution has occurred. We’ve suggested that maybe epigenetics has a significant role in the evolutionary process,” Skinner says, “that the environment can actually influence the process, independent of (gene) sequence mutation, to develop a trait.”
Skinner is saying that acquired characteristics can be inherited — an idea advanced by Jean-Baptiste Lamarck and others in the late 18th century and subsequently dismissed out of hand by ultra-Darwinists. To be clear, though, this neo-Lamarckian idea isn’t quite what Lamarck had in mind. He thought a giraffe’s neck would stretch as it nibbled the highest leaves from a tree in the African savanna and then that longer neck would show up in the next generation. The neo-Lamarckian, epigenetic version of the story maintains that some changes — particularly those occurring during early development — can enter the germline and be passed to the next generation. But these changes don’t affect genes; they are made in the complex epigenetic scaffolding that supports DNA. This may give organisms more flexibility in adapting to environmental changes, and it is certainly a factor in disease processes.
There are many diseases that clearly have a genetic component, including bipolar disorder, hemophilia and cystic fibrosis. But once scientists acquired the ability to search the genome for the flawed genes that led to these diseases, they discovered that just having the gene doesn’t mean you’ll get the illness. Even the breast cancer genes that confer horrifyingly high risk on women who carry them don’t absolutely doom those women to breast cancer, and many people who don’t have any of the known risk factors get cancer. So scientists began to consider forces affecting not the gene itself but gene expression — whether and when genes release the instructions that tell cells when to make proteins and what kind to make.
Even though every cell in your body carries a complete set of your DNA, not every cell is identical in function. To do their job, your liver cells crank out just the proteins livers need. They’re not making myelin for nerve sheaths, are they? You hope not. And the elegantly orchestrated switching of genes on and off is developmental biology in a nutshell — the essence of the embryo’s astounding journey from a fertilized egg to a fully formed newborn.
Until very recently, however, no one was certain that the environment could change how and when the switches were thrown, not just for an individual but for his or her descendants as well.
“There is starting to be a huge body of literature on environmental exposures in development, and probably the mechanism of (their effects) is going to be altered epigenetic programming,” says Jerrold Heindel, scientific program administrator for the Division of Extramural Research and Training at the National Institute of Environmental Health Sciences. “Now we have to discover how relevant it is to the human situation.”
It is likely to be relevant in some way. Epigenetic processes appear to be highly conserved — that is, they appeared early in the evolutionary process and have been carried through many branchings of the tree of life. A highly conserved pattern tends to work in the same way in many organisms, so animal studies involving conserved elements tend to be relevant to humans.
Epigenetic Toolbox
Bits and pieces of epigenetic discovery have made it into the popular press over the last decade, leading to valiant attempts to simplify the inherently complex chemistry of gene activation and suppression. As the Columbia Journalism Review recently noted, analogies have run the gamut: Epigenetics is the software to DNA’s hardware; epigenes are electric switches that turn genes on and off; epigenes are volume knobs that regulate the expression of genes; and so on. The addition of inheritability to the epigenetic tangle makes simplification a more difficult journalistic task.
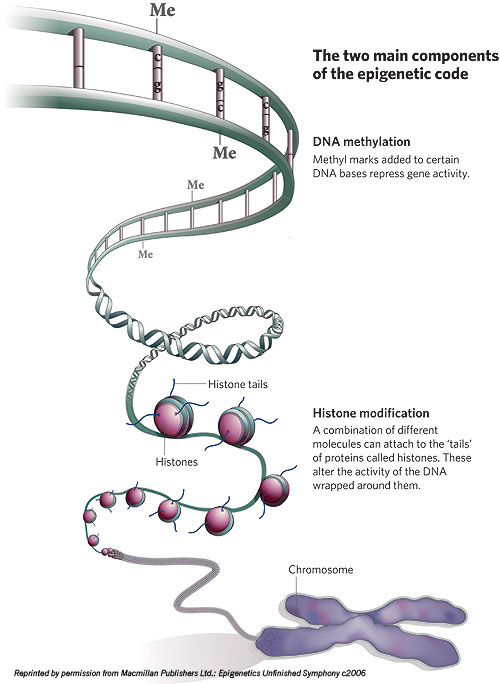
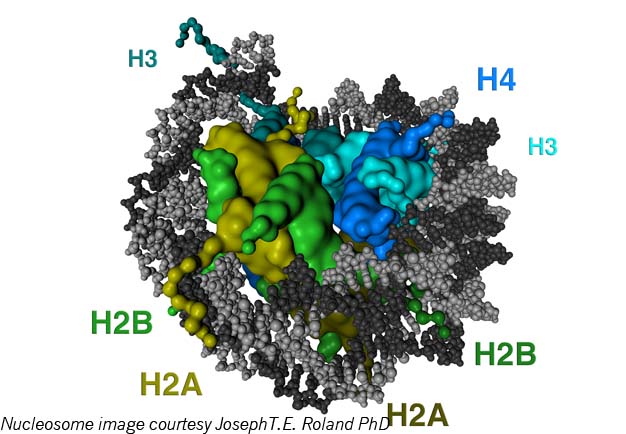
Let’s try another image: Epigenetic elements are a sort of wetware ult.aspx” target=”_blank”>Lego system. There are small protein blobs called histones that clump in groups of eight. Histones have little projecting bits, histone tails, to which several types of molecules (including the methyl, acetyl and phosphoryl groups, for those who are chemically conversant) can attach. DNA wraps a few times around each histone eight-pack; the resulting unit is called a nucleosome. Nucleosomes chain together by lengths of what is known as “linker DNA” into a form often described as “balls-on-a-string” and termed chromatin. Chromatin compresses in complicated folds, eventually fitting into the x-shaped forms of chromosomes inside the cell nucleus.
It’s thought that the epigenetic configurations of DNA within cells evolved because the DNA strand, nearly 6 feet long if stretched end to end, must be packed very carefully to fit into a nucleus inside a cell, itself invisible to the naked eye. The tightness of the DNA coils around the histones, along with the chromatin compression and packing, strongly influences gene activity: The looser the packing, the easier it is for various molecules to get access to genes to prompt their activation; the denser the packing, the harder it is for molecules to gain entry and the more genes are silenced.
During replication, the epigenetic pattern contained in the histone packing supporting the DNA double helix can be transcribed right along with the copying of the actual DNA. If methyl or other molecules are present where they shouldn’t be or absent where they should be, cancer and other diseases may arise.
Chemicals as Culprits
Suspicion that chemicals could cause damaging and possibly inheritable epigenetic patterns surfaced as scientists began to unravel the mystery of diethylstilbestrol, or DES. Doctors prescribed DES, a synthetic estrogen, to as many as 4 million pregnant women between 1938 and 1971 on the (incorrect) theory that extra estrogen would prevent miscarriage. By the early 1970s, it was evident that, after puberty, many daughters of these women, exposed to DES in the womb, contracted a vaginal cancer called clear cell adenocarcinoma.
During the 1980s and 1990s, researchers like Retha Newbold of the National Institute of Environmental Health Sciences and John McLachlan, now at Tulane University, became intrigued with this demonstration that a prenatal exposure could lead to an adult-onset disease. At first, the epigenetic effects seemed to be confined to cell replication within individuals, but DES research also hinted at multigenerational transmission of disease patterns. In 1998, Newbold and McLachlan reported increased tumors in the granddaughters of DES-exposed mice. The Gore-Crews-Skinner studies offer stronger evidence of the transgenerational phenomenon — that is, effects reaching beyond the offspring of exposed mothers into generations never directly in contact with the epigenetic agent.
Although the prospect of an agricultural or industrial chemical or a drug inflicting severe health consequences indefinitely into the future is worrying, it was not the point of the vinclozolin experiments conducted by Gore, Crews and Skinner. They were more interested in establishing the principle that an epigenetic mechanism could persist without alteration through several generations.
For their experiment, Skinner says, his team did not do classic toxicology experiments to determine the level of vinclozolin exposure necessary to cause the effects seen on rats in his experiments. They used high doses unlikely to be found in the environment. “The level of vinclozolin in the environment could be safe,” he says. “We don’t know.”
Still, the experiment showed that a great-great-grandmother rat’s exposure to a particular fungicide had an inherited impact on her great-great-grandson. “Clearly, the industry is now aware of the fact that it’s not just the individual exposed early in development (who can be affected),” Skinner notes. A study of vinclozolin published in the April 2008 issue of Reproductive Toxicology found no effect on sperm counts and no transgenerational effects. The study authors work for BASF, the manufacturer of vinclozolin. The study used a different strain of rats, and its highest vinclozolin doses were equal to the lowest vinclozolin doses in the Skinner study.
In their experiment, Gore, Crews and Skinner found that the females from the contaminated lineage did not pass the reproductive problem caused by vinclozolin to their daughters; therefore, if the females select uncontaminated mates, this curse can be lifted from future generations. But if the epigenetics of an entire population has been affected in a similar way, there will be no means to overcome the extreme challenge to survival posed by this sort of transgenerational health disaster. “In those animals,” Crews says, “the population is more or less doomed.”
Little is known about whether humans select mates in a way even slightly similar to the way lab rats do. But we know that certain chemicals have made their way into nearly every organism on Earth, including humans. The Centers for Disease Control and Prevention’s Third National Report on Human Exposure to Environmental Chemicals (2005) shows that Americans have in their bodies a wide array of pesticides, industrial chemicals and heavy metals. Long after they were banned, PCBs and DDT are still found in Arctic mammals, as is the still widely used flame retardant tetrabromobisphenol A, according to the Canadian Arctic Contaminants Assessment Report II, published by the government of Canada. Some of these chemicals are known to be hormone-system or metabolism disrupters and carcinogens. If they turn out to have transgenerational effects, humans may be as much at risk as other species. This particular “if” is a big one; most of these substances have not been tested for inheritability.
It’s Not My Fault After All
It may be true for Vegas, but does what happens in the womb stay in the womb, or does development equal destiny?
J.P. Barker proposed his developmental-origins hypothesis in 1997 after observing that low birth weight correlated strongly with heart disease in adult life. Barker’s idea has been supported many times over; studies show positive relationships between low birth weight and high blood pressure, type 2 diabetes, chronic lung disease and many other diseases. Of increasing concern are the metabolic disorders. Research has shown that maternal stress, particularly when caused by persistent under- or malnutrition, prompts the fetus to become “thrifty” — that is, to prepare itself for an environment in which it will not get enough of the right things to eat. When the baby is born into a world of plentiful food, he or she can’t adjust to the new reality. Though underweight at birth, such babies often later undergo a spurt of unhealthy weight gain.
Excess weight is an epidemic problem in the United States. About two-thirds of the population is either overweight or obese, according to the National Institutes of Health Weight-control Information Network. Bruce Blumberg of the University of California, Irvine has been investigating whether prenatal exposure to environmental chemicals that he calls obesogens is a factor in the epidemic. Previous research has shown that some endocrine-disrupting chemicals that act like estrogens, including bisphenol A, nonylphenols and organotins, can stimulate the differentiation and proliferation of fat cells. Blumberg thinks that epigenetic mechanisms likely are at work.
Blumberg studies an organotin called tributyltin (TBT), a persistent and bioaccumulative endocrine disrupter used as a fungicide on crops like pecans, celery and potatoes and to kill barnacles encrusted on ship hulls. TBT has crashed some populations of marine snails by causing females to develop male sexual organs. Closely related organotins are used in PVC plastics, including domestic water piping. In animal experiments with TBT, Blumberg has observed changes in the development and location of fat cells. In an April lecture sponsored by the Oregon Environmental Council in Portland, Blumberg said that the fat cells of mice exposed prenatally to TBT already contained fat at birth, whereas those of the untreated animals were mostly empty (the normal condition at birth). And after African clawed frogs were exposed to TBT, their testes contained fat cells instead of sperm. Unpublished work by Blumberg’s lab suggests that TBT shunts a type of stem cell onto a fat cell track, apparently at concentrations equivalent to those of natural hormones.
Although Blumberg hasn’t found an epigenetic smoking gun for obesity yet, he says, “It’s the only logical explanation for how we can expose animals while they’re still in the womb and have them live a changed life thereafter. There’s no other way you can do that except by epigenetics.”
A Two-Way Street
If you haven’t already dropped this magazine and run away screaming, please keep reading. There are reasons for optimism in the tiny, tangled world of epigenetics. For one thing, epigenetic processes are by their very nature flexible, unlike genes, which by comparison are resistant to change.
A striking example of epigenetic plasticity is the work of a team led by Randy Jirtle of Duke University, published in the Proceedings of the National Academy of Sciences in August 2007. Jirtle dosed pregnant mice with bisphenol A (BPA), a controversial ingredient in plastics that is known to be an estrogen disrupter. The exposed pups developed yellow fur instead of brown and became obese; BPA had altered methylation patterns in the pups’ genome at points determining coat color and metabolism.
But further experiments showed that if the BPA-exposed pregnant rat’s diet included extra folic acid, vitamin B12, choline and betaine, the effects of BPA on her offspring were blocked. This shows that naturally occurring chemicals and nutrients, not just industrial chemicals, can affect epigenetic patterns — and the effects can be positive.
Some research suggests that not just disease processes but behavior may be controlled by epigenetic mechanisms. Work published in 2005 by Moshe Szyf, Frances Champagne and their colleagues at McGill University in Montreal indicates that anxiety and the ability to cope with stress in adult rats depends directly on how much licking and grooming they got from their mothers as pups. The right amount of attention methylates the gene for a glucocorticoid receptor expressed in the brain. Anxious, stressed-out rats with nurturing deficits experience relief when dosed with methionine, a chemical related to the popular supplement SAM-e. The McGill studies support the idea that epigenetic patterns controlling some behaviors are themselves shaped by maternal behavior and can be altered pharmacologically.
The Szyf group also published a report this year in the Public Library of Science that compared the brains of 13 suicides who had been abused as children with 11 nonabused suicides. They found altered methylation of a gene that regulates protein synthesis in the abused victims’ hippocampus, an area of the brain involved in mood regulation.
According to Crews, epigenetics may also have a bearing on many human diseases that produce inappropriate or dangerous behaviors, such as autism, Alzheimer’s and schizophrenia. In research being prepared for publication that investigates the epigenome of the amygdala, a brain region active in emotions, Crews says he has found evidence that “there is a significant change in genes that have been implicated in affective disorders.”
Clearly, epigenetic change caused by environmental factors can result in disease and disorders. But research is also starting to show that the environment can be manipulated to treat disease regulated by epigenetics.
Prevention or Cure
At the moment, epigenetics seems like a minefield through which it is a miracle that any organism can travel to reach functional maturity. Gestation, in particular, seems such a delicate phase that perhaps we should return to the Victorian era when pregnant women, at least the rich ones, were treated like heirloom china cups. Maybe we should create maternity spas where nothing is stored in plastic, all the air and water are filtered, all the food is organic and someone else does the vacuuming — with a HEPA filter. But that wouldn’t do anything for the already born, which raises the question: If you’re an adult, is it too late?
Actually, in the realm of environmental impacts on epigenetics, adulthood confers some advantages. If you’ve gotten that far in life, your body is probably pretty functional. Toxic exposures, at least at relatively low concentrations usually found in the environment, tend to hit the young far harder than they do adults. But it’s still unnerving to think of one’s cells as ticking time bombs, preparing to fulfill their tragic destiny on, say, the day after your retirement party. A phenotype sensitized by a prenatal imbalance may lie dormant for a long time and then go out of whack after a re-exposure to the original sensitizing agent, such as estrogen, radiation or cigarette smoke. And at several points over a lifespan the fantastically complex processes of cell reproduction become very dangerous: from conception through about age 2; during puberty; and in late adulthood, including menopause for women. These are the ages when the body must achieve balance while hormone levels fluctuate and cells tend to proliferate.
None of the studies discussed here constitutes conclusive proof of a new paradigm that will sweep away what came before, but if further research confirms and extends the findings to date, the landscape of disease treatment and prevention will change radically. Many researchers and drug companies are working on epigenetic treatments, especially for cancer. In most cancers, methyl molecules are missing from their expected locations, but sometimes the opposite is true. (This is one reason not to tank up on high-dose nutritional supplements that encourage methylation.)
Cancer researchers are greatly interested in a group of genes called tumor suppressors because if they go awry, a person’s cancer risk escalates. Stephen Baylin, a researcher at the Johns Hopkins University School of Medicine, has been studying DNA methylation’s role in cancer for 20 years. He and others have found that in precancerous cells, tumor suppressor genes are silenced. “The loss of that expression is an alternative to mutations for some of the genes,” Baylin says. This silencing is “probably playing a fundamental role in the onset and progression of cancer. Every cancer that’s been examined so far, that I’m aware of, has this (pattern of) methylation.”
At the moment, few proven therapies for renegade methylation are available. Baylin points to the success of one drug, azacytidine, that delays the progression of a leukemia precursor, myelodysplasia, apparently by re-methylating silenced genes. “It’s not clear yet whether all of that therapeutic response is due to (epigenetics),” Baylin says, “but in the test tube it turns all of these genes back on. Some patients in the trial period have been on the drug for 10 years and so far haven’t had detrimental effects. It’s much less toxic than standard chemo.”
The potential for epigenetic disease therapies is attracting major investment. Danny Reinberg, a professor of biochemistry at New York University School of Medicine and a Howard Hughes Medical Institute investigator, has just put together a new company called Constellation, initially capitalized at $32 million. “Our goal is to target the enzymes that catalyze lysine methylation and lysine demethylation for cancer and other diseases,” Reinberg says. He estimates it will take approximately 15 years to bring a drug that will do this to market.
Few researchers envision the field reaching a point where heart disease, diabetes and the other chronic scourges of humankind will be cured by taking a sort of epigenome-resetting pill, but most expect that, at some point, individual epigenomes can be assayed early and used as a guide for preventive measures throughout a lifespan.
The emergence of epigenetics, fetal origins of disease and endocrine disruption theories bolsters the argument for adopting the precautionary principle when regulating the environment. Of the approximately 80,000 industrial chemicals on the market today, only a small percentage have been tested for safety, and those have mainly been assayed for crude toxicological effects, not for their long-term, low-level or developmental influence. As Crews notes, “We have no idea the extent to which there’s been damage in humans” from the constant bath of chemicals they encounter.
And Skinner asks, “How much of the disease we see in our society today is transgenerational and more due to exposures early in life than anything else?” The answer will likely apply equally to all inhabitants of the planet, all the Betsies and their beaux on land and sea, human, rodent and other.
Reading Room
A selection from the research reports reviewed for this article:
Crews, David, Andrea C. Gore, Timothy S. Hsu, Nygerma L. Dangleben, Michael Spinetta, Timothy Schallert, Matthew D. Anway, and Michael K. Skinner. 2007. “Transgenerational Epigenetic Imprints on Mate Preference.” Proceedings of the National Academy of Sciences, Vol. 104, No. 14, pp. 5942-5946. Retrieved from http://www.pnas.org/cgi/reprint/104/14/5942.
Dolinoy, Dana C., Dale Huang, and Randy L. Jirtle. 2007. “Maternal Nutrient Supplementation Counteracts Bisphenol A-Inducted DNA Hypomethylation in Early Development.” Proceedings of the National Academy of Sciences, Vol. 104, No. 32, pp. 13056-13061. Retrieved from http://www.pnas.org/cgi/reprint/0703739104v1.
Gross, Liza. 2007. “The Toxic Origins of Disease.” PLoS Biology 5(7): e193. Retrieved from http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0050193&ct=1.
Tabb, Michelle M., and Bruce Blumberg. 2006. “New Modes of Action for Endocrine-Disrupting Chemicals.” Molecular Endocrinology, Vol. 20, No. 3, pp. 475-482.
Weinhold, Bob. 2006. “Epigenetics: The Science of Change.” Environmental Health Perspectives, Vol. 114. Retrieved from http://www.ehponline.org/members/2006/114-3/ehp0114-a00160.pdf.
Sign up for our free e-newsletter.
Are you on Facebook? Become our fan.
Follow us on Twitter.




