Access to birth control just got significantly easier in four African countries. Sayana Press, a new pre-filled, single-dose contraceptive was introduced Burkina Faso in early July, and within the next few months it will be available in Niger, Senegal, and Uganda, too. It’s unique because of how it was developed and introduced, and because of what it could mean for women in countries where birth control isn’t widely available or is stigmatized.
Sayana Press is a combination of two things—a drug and a device—that are both already available. It’s Depo-Provera, a widely used, long-acting contraceptive, in a Uniject device. Uniject, which looks like a tiny golf ball on a tee, is a single-use, pre-filled injection system developed by Becton Dickinson. To use it, you crack a tiny one-way seal, and then squeeze a bubble to dispense the drug. It’s previously been used to administer tetanus and Hepatitis B vaccines.
“In the big picture right now, what we’ll call the global institutional buyers like USAID, are responsible for a really large share of contraceptives available in developing countries.”
Putting Depo-Provera in the Uniject solves several contraceptive issues endemic to sub-Saharan Africa. Access is a big one. Depo-Provera was previously administered with a vial and syringe. That meant that women had to go to a clinic to get it, and, because it was a two-piece system, there could be a mismatched supply of drugs and needles, a common problem in low-resource areas. Health workers might have the drug at their clinic, but no way to administer it, or visa versa.
Uniject also has a single-use needle, which alleviated the problem of needle sharing in places where HIV and AIDS are widespread. Plus, Sayana Press is discreet and each injection lasts for three months—another big factor in developing countries. Women who want it don’t need to go to a clinic to get it, and community-based health workers in the field can hand it out for future use.
Path, a non-profit that works on global health projects ranging from epidemiology to immunization, has been developing Sayana Press since the 1980s. Sara Tifft, Path’s global program director for the Sayana Press project, says that they think easy access to contraceptives are crucial to individual, family, and community health in developing countries. They had identified a specific need for easy-to-administer birth control that was also easy to transport, so that it could be brought in to and used in remote parts of the developing world. She says that almost a quarter of married women in Burkina Faso wanted access to birth control that they didn’t have.
FAMILY PLANNING IS A global issue that takes very different forms in different countries—America included—because of policy, access to health care, and social norms. Path’s Sara Tifft says Sayana Press doesn’t really make sense in the United States, for instance, because American woman have to get prescriptions for contraceptives from their health care providers. It’s just as easy to get a Depo-Provera shot when you go to see your doctor.
In Africa, and in other developing countries, the issues are different. There, access, education, and ease of use are the primary barriers to entry, not health insurance.
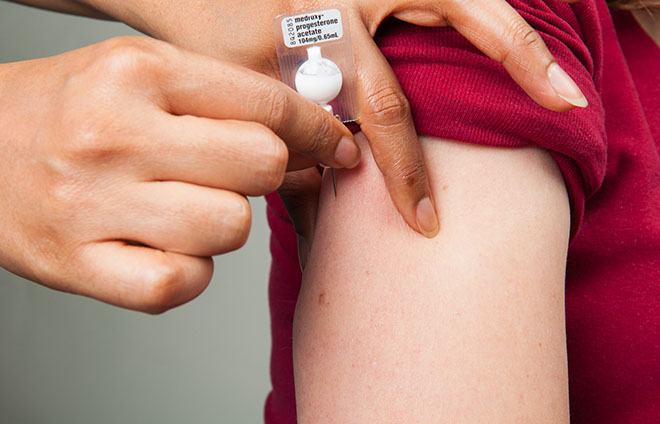
Sayana Press Injectable Contraceptive. (Photo: UNICEF)
Sayana Press, and the idea of a self-contained, single use contraceptive gained steam after the 2012 London Summit on Family Planning. There, 150 partner groups, from governments to private sector funders, agreed to help with the project. “The whole initiative was driven by trying to raise the tide level for all the boats,” Tifft says. “There was a lot of buy in because everyone shares common goals.”
The number of groups involved made development and implementation of Sayana Press unique, Tifft says. Pfizer developed the drug and conducted clinical trials while the majority of the funding came from the Bill & Melinda Gates Foundation, the United States Agency for International Development, and the U.K.’s Department for International Development.
On the ground, implementation varies depending on the country. In Niger and Burkina Faso, the Ministries of Health handles training, while in Senegal and Uganda, NGO partners do it on behalf of the government. Collaborative resources and varying strategies make the project complicated, but, as with other cross-border global health issues, they’re also what make it possible.
Sara Tifft, Path’s global program director for the Sayana Press project, says that they think easy access to contraceptives are crucial to individual, family, and community health in developing countries.
To roll out a project like Sayana Press, first you need a drug and then you need to get it approved in the countries where it’s going to be used. “You can’t procure and ship until you have regulatory approval,“ Tifft says. Sally Jacobs, senior director of communications at Pfizer, says that Depo-Provera has been available since 1961, when it was first approved in Canada. The drug itself is approved in 124 countries, but they had to get it approved specifically to be administered in the Uniject device. Sayana Press is currently approved in 19 countries in Europe, Asia, and Africa. Regulation varies significantly from country to country, even in places where Depo-Provera and Uniject had both been used before, which is one of the reasons why Path can’t implement a blanket global strategy.
Once a drug is approved, rollout strategy, education, and distribution are addressed on a country-by-country basis. Burkina Faso is implementing the drug first, Tifft says, because it was the first country to get organized.
Contraception is especially tricky because of the stigma attached to it—from government policy to the way a husband might perceive his wife’s use of birth control. It’s unique in the public health space because it’s something that you need a drug for that’s not a disease. “People are making a choice when they use contraceptives,” Tifft says. “The ability to get pregnant is worldwide, it’s endemic to all women.”
That’s part of why Path and the other partners, like Gates and the United Nations’ Population Fund, think that Sayana Press is important. They don’t want availability or usability to be a barrier to women getting birth control. “In the big picture right now, what we’ll call the global institutional buyers like USAID, are responsible for a really large share of contraceptives available in developing countries,” Tifft says.
After Africa, Path is working to make Sayana Press available in South Asia, another area of the world where access to contraceptives in rural areas is fraught, and where family planning could greatly improve quality of life. ‘This is totally need driven,” Tifft says.




