IT WAS THE WORST OUTBREAK OF DENGUE FEVER IN AUSTRALIA IN 60 YEARS. More than 1,000 people were stricken by the potentially fatal, insect-born infection in a 2009 epidemic that swept through the beach towns of northern Australia with lightning speed.
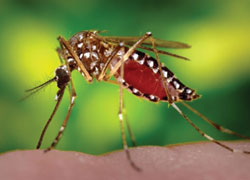
The Aedes aegypti mosquito can transmit a range of diseases, including Dengue fever
“Right over there is where it started,” Scott Ritchie says, pointing to a green clapboard cottage perched on stilts to avoid floods. It’s an overcast but warm April day in a quiet, palm-tree-dotted suburb of Cairns, a jumping-off point for expeditions to the Great Barrier Reef. Ritchie, a medical entomologist, explains that the Aedes aegypti mosquitoes that transmit dengue like to congregate in the cottage’s aboveground cellars, which provide a nice, damp breeding ground. The summer of 2009 was especially hot and wet, and the strain of dengue especially fast-incubating—perfect conditions for an epidemic. The first patient contracted the virus in Indonesia and never sought medical care. “By the time we detected it,” sighs Ritchie, making a sweeping gesture with his hand, “it had spread throughout this whole area.”
With his brush cut and jaunty brio, the lanky, jeans-clad, American-born scientist seems like a high school football coach. But Ritchie is part of an Australian research team that is developing a potentially revolutionary new method to defeat dengue—by engineering the very insects that carry the disease.
A handful of other researchers in the U.S. and Europe are also working on techniques to biologically modify bugs to fight diseases. If they succeed, they could create an entirely new way of stopping not only dengue but other insect-borne scourges, such as yellow fever, West Nile virus, and malaria. And stopping these diseases has never been more urgent.
In North America, mosquitoes are pesky critters kept mostly out of our lives by window-screens and air-conditioning. But in hotter climes, especially in overcrowded mega-cities in Africa, Asia, and South America, the tiny insects are airborne angels of death, delivering lethal pathogens that sicken 400 million people and kill at least one million annually. Worse, mosquitoes are becoming resistant to the latest generation of pesticides.
Decades of costly research into vaccines for ailments such as malaria have had limited success. And the number of people exposed to mosquito-delivered afflictions is expected to soar into the billions as the planet heats up and mosquitoes extend their range to newly warm habitats—including in the United States.
Dengue in particular is a growing threat. The World Health Organization calls dengue the most serious of the “re-emerging” diseases—infections that were once thought conquered, but have come roaring back since eradication campaigns that carpet bombed breeding grounds with DDT were abandoned in the early 1960s. Dengue’s incidence has spiked 30-fold since then; it sickens as many as 100 million people every year in scores of countries. Normally, a dengue infection feels like a bad case of the flu, causing chills, fever, headaches and such crippling joint pain that it’s sometimes called breakbone fever. But the more serious form, dengue hemorrhagic fever, can be fatal, triggering massive internal bleeding by eating away blood vessels and internal organs, and there are no vaccines or treatments.
Thanks to extensive eradication campaigns, dengue was essentially eliminated in the Western Hemisphere by the 1960s. But it began reappearing in the U.S. in the 1990s; serious outbreaks in Florida, Puerto Rico, and along the Texas-Mexico border between 1995 and 2005 sickened 10,000 people. Across the Western Hemisphere, 900,000 people were infected in 2007. “We desperately need additional tools for dengue,” says Stephanie James, director of science with the Foundation for the National Institutes of Health.
BIOLOGICALLY ALTERING BUGS ISN’T ENTIRELY NEW; it’s been done for nearly half a century to protect crops. In the U.S., when a troublesome pest is spotted, Department of Agriculture technicians round up thousands of them and blast them with radiation, sterilizing them. Then the males, which are slightly smaller than the females, are separated out and released to mate with wild females. The union produces infertile eggs, which eventually wipes out the wild populations. It can take months for this strategy to take root, because the sterilized males don’t compete as effectively for mates as their wild counterparts. Nonetheless, this approach was used to vanquish the tsetse fly in Zanzibar in the 1990s. In California’s farmlands, millions of sterile Mediterranean fruit flies are dropped from airplanes every week. And in Arizona’s cotton fields, the USDA is testing the process on the pink bollworm, a moth caterpillar that munches on cotton.
It’s only recently, however, that scientists have begun experimenting with using this technology to combat human diseases. Now, after more than a decade gestating in the lab, some bug-engineering tactics may be ready for prime time.
“THERE ARE TWO GENERAL APPROACHES,” says Anthony James, a University of California, Irvine, geneticist and a pioneer in the development of technology to alter mosquito DNA. “Either self-limiting, in which the mosquito population is driven to extinction, or self-sustaining,” in which the altered bugs take over, thereby halting the spread of disease.
Some researchers, including the Australians and groups at Johns Hopkins University in Baltimore, are smuggling a hitchhiking bacteria into the dengue-carrying mosquitoes that prevents them from passing on the virus. A British team is tinkering with DNA to either significantly reduce the lifespan of malaria-carrying mosquitoes (known as Anopheles) or kill females when they are just embryos. Either method would cause a population crash. In James’s lab in Southern California, scientists are working on similar techniques.
What these methods all share is the promise of blanket protection: they can theoretically kill or disable mosquitoes that insecticides miss—bugs nesting in hidden pools of water, for instance, or that lay eggs in storm drains or flower pots. What’s more, bioengineering bugs is relatively cheap and doesn’t require toxic pesticides.
“These approaches have the potential to be huge, and to reach remote populations,” says Stephanie James of the Foundation for the National Institutes of Health, a joint venture between the NIH and the Bill and Melinda Gates Foundation, which funds most of these projects. The strategy should work whether people sleep under bed nets, have access to vaccines, or live in mosquito-proof houses.
Scott Ritchie in his lab in Cairns, Australia.

AT RITCHIE’S LAB AT JAMES COOK UNIVERSITY, near Cairns, on a densely foliated campus surrounded by forested mountains, the team goes through the tedious process of infecting mosquitoes with a bacteria called Wolbachia to prevent the bugs from passing on dengue. Wolbachia is an innocuous microbe present in 70 percent of insects. Scientists don’t understand exactly why this Trojan horse stops bugs from transmitting dengue—they just know it works. “It’s like a dengue vaccine for the mosquito,” Ritchie explains.
The first step is coaxing female mosquitoes to lay eggs, which they only do after eating a blood meal (the researchers take turns getting bitten by dengue-free mosquitoes). Only females drink blood, so only females transmit the diseases. Once sated, the mosquitoes are put in test tubes, where they lay their eggs within about an hour. The eggs, which look like tiny coffee grains, are placed on swatches of a red feltlike material. Then, scientists use an ultrathin needle to inject the delicate mosquito eggs with Wolbachia, which insinuates itself inside the bug.
When the eggs mature into larvae, they’re incubated in mesh-covered ceramic pots and stored on stainless steel racks that line the lab. Once the larvae hatch, the cycle starts again. When the infected female mosquitoes reproduce, they pass the bacterium on to their offspring. And if an uninfected female pairs up with an infected male, says Ritchie, “he shoots blanks.” The eggs fail to hatch, giving the infected insects an extreme survival advantage.
In 2011, Australian researchers released about 300,000 infected bugs in two Cairns suburbs that were the site of the 2009 dengue outbreak. Over the following three months, the researchers trapped mosquito eggs in the field, then hatched them in the lab and used a genetic diagnostic tool to determine the mosquito’s species and whether it contained the Wolbachia bacteria.
A researcher releases mosquitoes infected with the Wolbachia bacteria into the wild in northeastern Australia.

In less than two months, 100 percent of mosquitoes in one community and 80 percent in the other carried the Wolbachia bacteria. Within five months, the bacteria infiltrated the entire mosquito population and were affecting bugs in surrounding neighborhoods. “Those results exceeded all expectations,” says Scott O’Neill, an entomologist at Monash University in Melbourne who heads the Australian team.
The next step is to confirm that this strategy actually stops the bugs from spreading dengue. In lab cages, O’Neill’s team fed mosquitoes blood containing the dengue virus, and then infected them with a slightly different strain of Wolbachia, called wMelPop-CLA. It was completely successful in preventing mosquitoes from transmitting dengue to their offspring. In January of this year, researchers began releasing 200,000 mosquitoes infected with this new bacteria strain in two other Cairns suburbs. The latest results show infection rates of about 80 percent and climbing. But because northern Australia only has sporadic dengue outbreaks, the true trial will be in places like Vietnam, China, and Indonesia, where dengue fever is endemic. The Australian team members are awaiting government approvals to let them release lab-altered mosquitoes in those countries.
IN SOUTHERN CALIFORNIA, geneticist Anthony James, an ingratiating 6-foot-4-inch charmer with a megawatt smile, shows me around his cluttered lab at UC Irvine, housed in two large rooms about the size of a basketball court. He introduces me to one of his colleagues, who sits at a lab bench, hunched over a microscope as she injects DNA into mosquito embryos. The bench behind her is lined with the familiar ceramic pots containing newly hatched mosquitoes crawling across the mesh coverings.
James and his team are pursuing two lines of attack. One involves injecting male Aedes aegypti mosquitoes with a lethal gene that will kill them unless they’re given the antibiotic tetracycline. That way, researchers can breed generations of these altered bugs in the lab and keep them alive with antibiotics until they’re released. In the wild, the mosquitoes live long enough to mate, but their offspring die within a few days—and eventually the population collapses. James has worked with a U.K.-based biotech company called Oxitec that is sponsoring field trials in the Cayman Islands, Brazil, and Malaysia. In Oxitec’s most recent tests in the Cayman Islands, in 2010, the group released 3.3 million transgenic male mosquitoes, which mated with wild females that then produced sterile larvae. Eighty percent of the indigenous mosquito population was wiped out within six months.
But James wasn’t convinced this approach could become self-sustaining in the wild. So in a tiny village on the outskirts of Tapachula, Mexico, where dengue is rife, James tested a slightly different strategy. He genetically engineered male mosquitoes so that when they mated with wild females, their female offspring inherited a genetic defect that weakened the muscles that powered their wings. Unable to fly, the new females couldn’t spread the disease or even mate. Researchers came up with the tactic when they realized one region of the mosquito DNA regulated the development of flight muscles for female larvae but not for males—which meant it could be easily manipulated to wipe out females.
In experiments with colonies at the Mexican research station, James and his researchers drove the mosquito population to extinction within a few generations. “The flightless females work like a genetic insecticide,” says James. He hopes to release the genetically engineered males to the open field in the near future.
In the meantime, James has a bigger target in his sights: the Anopheles family of mosquito that spreads malaria.
Female mosquitoes pick up malaria-causing parasites when they feed on the blood of an infected human. The parasite gestates inside the insect’s gut, then migrates to the mosquito’s salivary glands, where it is passed to another victim the next time the mosquito feeds. Mice, though, don’t get human malaria; their immune systems quash the parasites. James and his collaborators were inspired by the work of cancer researchers, who were using antibodies to rally the immune system to fight cancer. So they identified the genes that help mice ward off malaria. With those as a model, James was able to build an artificial gene that bolsters a mosquito’s immune system, killing the virus inside the insect. “We’re testing to see if the gene gives us 100 percent knockout of the parasites,” says James, “and can break the transmission cycle.” (It’s conceivable that a similar tactic could work on humans, James says, but that’s still a long way off.)
Yet while the potential gains are huge, critics fear the unpredictable consequences of releasing altered bugs into the wild. Nature abhors a vacuum: if we wipe out the mosquitoes, will another, potentially more troublesome pest take their place? And mosquitoes pollinate thousands of plants, and are themselves food for birds, fish, and other bugs; what happens if they disappear? Altered mosquito genes might also migrate to other insects, causing the destruction of gnats, flies, and other creatures that are vital to ecosystems.
So far, these scenarios haven’t come to pass, according to John Mumford, an entomologist at the Centre for Environmental Policy at Imperial College in London. “While there are environmental concerns,” he says, “there has been no indication that anything lasting has happened after [researchers] stopped releasing these modified insects.”
Because the regions that see the most devastation from insect-borne diseases are in developing countries, scientists and biomedical ethicists also worry that field trials could prey on poor communities.
James and other researchers are well aware of these concerns. That’s why, when they set up the field laboratory in Tapachula, James bought the land, and spent more than two years working with local authorities, farmers, nurses, and residents to familiarize them with what the team was doing and why the research would help the area’s residents. Similarly, the Australians met frequently with suburban homeowners, health authorities, and tourism officials before launching the wMelPop-CLA field trials. “We purposely did it in our own country first,” says O’Neill, to avoid seeming exploitive.
Not all researchers are that conscientious. When Oxitec quietly released millions of altered bugs in the Cayman Islands, it had governmental approval—but hadn’t consulted with the locals. And confidential documents recently obtained by GeneWatch UK, a watchdog group that monitors genetic engineering, show that while Oxitec’s technology is supposed to prevent the sterile mosquitoes from surviving in the wild, a small percentage actually do.
Oxitec chief scientist Luke Alphey says these fears are misplaced, and that the company’s experiments are safe. Nonetheless, says Helen Wallace, director of GeneWatch UK, “It’s impossible to assess health or environmental risks if important information is concealed from public scrutiny. This document reveals a fundamental flaw in their technology which should have halted their experiments. We feel they’re pushing ahead just to commercialize the technology.”
Despite these misgivings, researchers believe the tiny insects buzzing around the enclosed greenhouselike arboretum adjoining Ritchie’s lab near Cairns may ultimately turn the tide in the fight against infectious diseases, especially in poor countries. “Dengue and malaria kill hundreds of thousands of children every year,” says O’Neill. “This is an urgent public health problem.” Solving that problem begins with small steps—perhaps as small as a single mosquito.





